Abstract
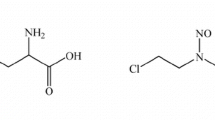
Stability and preclinical pharmacokinetics of isophosphoramide mustard (IPM), an active metabolite of ifosphamide, were investigated using analytical methods developed in this laboratory. For stability evaluation of IPM we used a rapid, high-pressure liquid chromatographic (HPLC) method by which IPM is analyzed directly from aqueous solutions without derivatization on a 10-μm C-18 reversed-phase column with theophylline as the internal standard. IPM in sodium phosphate buffers was found to undergo pH-dependent first-order degradations. At pH 7.4 and 38° C, the IPM solution showed a half-life of 45 min. A gas chromatographic-mass spectrometry (GC/MS) method for the analysis of IPM in plasma was also developed. This method utilized solid-phase extraction with deuterium-labeled IPM as the internal standard. The routine detection limit for the assay was 50 ng/ml with within-run and between-run coefficients of variation of 6% and 11%, respectively. By this method, stability of IPM in plasma and in RPMI 1640 tissue culture medium was evaluated, and its pharmacokinetics in the Sprague-Dawley rat following i.v. administration at 40 mg/kg were investigated. IPM was found to be more stable in these media, with half-lives in the range of 100 min. IPM plasma pharmacokinetics were found to decline monoexponentially with terminal halflives ranging from 6.8 to 18.7 min and total clearance between 6.0 and 18.3 ml/min. Plasma protein binding of IPM was found to be 55%, and the partition ratio between plasma and red blood cells of 4.9 to 1, respectively. Cytotoxicity of IPM to L1210 cells was evaluated, and the results indicated that the IC50 with 1-h and 4-h exposure was 33 and 15 μm, respectively. Based on these data, IPM plasma levels in the rat declined below the IC50 in about 1 h at this dose. More frequent dosing or infusion may be necessary to maintain adequate drug levels for antitumor activity when IPM is administered directly.
Similar content being viewed by others
References
Phelan ET, Vietti TJ, Valeriote FA, Coulter D (1981) Comparison of cytotoxic action of iphosphamide and cyclophosphamide. Anticancer Res 1: 149–154
Lewis LD, Meanwell CA (1990) Ifosfamide pharmacokinetics and neurotoxicity (letter). Lancet 335: 175–176
Sarosy, G (1989) Ifosfamide-pharmacologic overview. Semin Oncol 161: 2–8
Allen LM, Creaven PJ (1972) In vitro activation of isophosphamide (NSC-109724) a new oxazaphosphorine, by rat liver microsomes. Cancer Chemother Rep 56: 603–610
Norpoth K (1969) Untersuchungen zur oxydativen Umsetzung von Endoxan in vivo und in vitro. Thesis (Habilitationsschrift) Westphalian Wilhelm University, Münster
Hill DL, Laster ER, Struck RF (1972) Enzymatic metabolism of cyclophosphamide and nicotine and production of a toxic cyclophosphamide metabolite. Cancer Res 32: 658–665
Sladek NE (1973) Evidence for an aldehyde possessing alkylating activity as the primary metabolite of cyclophosphamide. Cancer Res 33: 651–658
Sladek NE (1973) Bioassay and relative cytotoxic potency of cyclophosphamide metabolites generate in vitro and in vivo. Cancer Res 33: 1150–1159
Connors TA, Cox PJ, Farmer PB, Foster AB, Jarman M (1974) Some studies of the active intermediates formed in the microsomal metabolism of cyclophosphamide and isophosphamide. Biochem Pharmacol 23: 115–129
Volcker G, Drager U, Peter G, Hohorst HJ (1974) Studien zum Spontanzerfall von 4-Hydroxycycolphosphamid und 4-Hydroperoxycyclophosphamid mit Hilfe der Dünnschichtchromatographie. Arzneimittelforschung (Drug Res) 24: 1172–1176
Takamizawa A, Matsumoto S, Iwata T, Katagiri K, Yamaguchi K, Shiratori O (1975) Studies on cyclophosphamide metabolites and their related compounds. Preparation of an active species of cyclophosphamide and related compounds. J Med Chem 18: 376–383
Colvin M, Padgett CA, Fenselau C (1973) A biologically active metabolite of cyclophosphamide. Cancer Res 33: 915–918
Struck RF, Kirk MC, Witt MH, Laster WR Jr (1975) Isolation and mass spectral identification of blood metabolites of cyclophosphamide: evidence for phosphoramide mustard as the biologically active metabolite. Biomed Mass Spectrom 2: 46–52
Fenselau C, Kan M-N, Billets S, Colvin M (1975) Identification of phosphorodiamidec acid mustard as a human metabolite of cyclophosphamide. Cancer Res 35: 1453–1457
DeVita VT, Adamson RH (1970) The metabolism of14C-cyclophosphamide: comparative drug metabolism studies on African and American patients with lymphosarcoma and Burkitt's tumor. Prog Antimicrob Anticancer Chemother 2: 218–225
Cohen J, Yao J, Jusko WJ (1971) Pharmacokinetics of cyclophosphamide in man. Br J Pharmacol 43: 677–680
Juma FD, Rogers JJ, Trounce JR (1980) The pharmacokinetics of cyclophosphamide, phosphoramide mustard and nornitrogen mustard studied by gas chromatography in patients receiving cyclophosphamide therapy. Br J Clin Pharmacol 10: 327–335
Wagner T, Heydrich D, Bartels H, Hohorst HJ (1980) Einfluss von Leberparenchymschäden, Niereninsuffizienz und Hämodialyse auf die Pharmakokinetik von Cyclophosphamid und seinen aktiviertem Metaboliten. Arzneimittelforschung, Drug Res 30: 1588–1592
Wagner T, Heydrich D, Jork T, Voelcker G, Hohorst HJ (1980) Über Blutspiegel und Urin-Ausscheidung von aktiviertem Cyclophosphamid und seinen Deaktivierungsprodukten beim Menschen. J Cancer Res Clin Oncol 96: 79–92
Wagner D, Heydrich D, Jork T, Voclcker G, Hohorst HJ (1981) Comparative study on human pharmacokinetics of activated ifosfamide and cyclophosphamide by a modified fluorometric test. J Cancer Res Clin Oncol 100: 95–104
Juma FD, Ogada T (1983) Pharmacokinetics of cyclophosphamide in Kenyan Africans. Br J Clin Pharmacol 16: 61–63
Power JF, Sladek NE (1983) Cytotoxic activity relative to 4-Hydroxycyclophosphamide and phosphoramide mustard concentrations in the plasma of cyclophosphamide-treated rats. Cancer Res 43: 1101–1106
Sladek NE, Doeden D, Power JF, Krivit W (1984) Plasma concentration of 4-Hydroxycyclophosphamide and phosphoramide mustard in patients repeatedly given high doses of cyclophosphamide in preparation for bone marrow transplantation. Cancer Treat Rep 68: 1247–1254
Sladek NE, Power JF, Grage G (1984) Half-life of oxazaphosphorines in biological fluids. Drug Metab Dispos 12: 553–559
Hong PS, Srigritsanapol A, Chan KK (1991) Pharmacokinetics of 4-Hydroxycyclophosphamide and metabolites in the rat. Drug Metab Dispos 19: 1–7
Hill DL, Laster WR Jr, Kirk MC, El Dareer S, Struck RF (1973) Metabolism of iphosphamide and production of a toxic iphosphamide metabolite. Cancer Res 33: 1016–1022
Struck RF, Dykes DJ, Corbett TH, Suling WJ, Trader MW (1980) Isophosphamide mustard, a metabolite of ifosfamide with activity against murine tumor comparable to cyclophosphamide. Br J Cancer 47: 149–154
Struck RF, Dykes DJ, Corbett TH, Suling WJ, Trader MW (1983) Isophosphoramide mustard, a metabolite of ifosfamide with activity against murine tumours comparable to cyclophosphamide. Br J Cancer 47: 15–26
Bryant BM, Jarman M, Baker MH, Smith IE, Smyth JF (1980) Quantification by gas chromatography ofN,N′-di(2-chloroethyl)-phosphorodiamidic acid in the plasma of patients receiving isophosphamide. Cancer Res 40: 4634–4738
Watson E, Dea P, Chan KK (1985) Kinetics of phosphoramide mustard hydrolysis in aqueous solution. J Pharm Sci 74: 1283–1292
Baker SK, Niazi S (1983) Simple reliable method for chronic cannulation of the jugular vern for pharmacokinetic studies in rats. J Pharm Sci 72: 1027–1029
Bolger MB, Muir KT (1986) An interactive graphics and nonlinear regression program for the analysis of experimental and simulated data. Proceedings of the 1986 Summer Computer Simulation Conference of the Society for Computer Simulation, 18–30. 7. 1986, Reno, Nevada.
Nayar MSB, Lin L-Y, Wan SH, Chan KK (1979) Gas chromatographic analysis of cyclophosphamide in plasma and tissues using nitrogen-phosphorus detection. Anal Lett 12 (B8): 905–915
Hardy RW, Moore MJ, Erlichman C, Soldin SJ (1987) Analysis of phosphoramide mustard by reversed-phase ion pair high pressure liquid chromatography. Ther Drug Monit 9: 221–226
Struck RF, Albert DA (1984) Blood levels of alkylating metabolites of cyclophosphamide in the mouse after intravenous vs. oral administration. Cancer Treat Rep 68: 765–770
Struck RF, Albert DA, Horne K, Phillips JG, Peng YM, Roe DJ (1987) Plasma pharmacokinetics of cyclophosphamide and its cytotoxic metabolites after intravenous vs. oral administration in a randomized crossover trial. Cancer Res 47: 2723–2726
Hadidi AH-FA, Idle JR (1988) Combined thin-layer chromatography-photography densitometry for the quantitation of cyclophosphamide and its four principal urinary metabolites. J Chromatogr 427: 121–130
Jardine I, Brundrett R, Colvin M, Fenselau C (1976) Approaches to the pharmacokinetics of cyclophosphamide (NSC-26 271). Quantitation of metabolites. Cancer Treat Rep 60: 403–408
Jardin I, Fenselau C, Appler M, Kan M-N, Brundrett RB, Colvin M (1978) Quantitation by gas chromatography-chemical ionization mass spectrometry of cyclophosphamide, phosphoramide mustard, and nor-nitrogen mustard in the plasma and urine of patients receiving cyclophosphamide therapy. Cancer Res 38: 408–415
Chan KK, Watson E, Hong SC (1985) The application of stable isotopes in antineoplastic drug research. In: Breimer DD, Speiser P (eds) Topics in pharmaceutical sciences 1985. Elsevier, New York
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York, pp 17–19
Pang KS, Cherry WF, Terrell JA, Ulm EH (1983) Disposition of enalapril and its diacid metabolite, enalaprilat, in a perfused rat liver preparation: presence of a diffusional barrier for enalaprilat into hepatocytes. Drug Metab Dispos 12: 309–313
Sladek NE (1973) Bioassay and relative cytotoxic potency of cyclophosphamide metabolites generated in vitro and in vivo. Cancer Res 33: 1150–1158
Colvin M, Brundrett RB, Kan MN, Jardin I, Fenselau C (1976) Alkylating properties of phosphoramide mustard. Cancer Res 36: 1121–1126
Fenselau C, Kan MN, Rao S, Myles A, Friedman OM, Colvin M (1977) Identification of aldophosphamide as a metabolite of cyclophosphamide in vitro and in vivo in human. Cancer Res 35: 2538–2543
Sharkis SJ, Santos GW, Colvin M (1980) Elimination of acute myelogenous leukemic cells from marrow and tumor suspensions in the rat with 4-Hydroperoxycyclophosphamide. Blood 55: 521–523
Gorin NC, Douay L, Najman C, Bailou C, Duhamel G (1982) Study of in vitro sensitivity of human leukemic cells and normal hematopoietic progenitors to 4-Hydroperoxycyclophosphamide (4HC): the interest for the preparation of antileukemic autologous bone marrow transplantation. Exp Hematol [Suppl 11]: 13
Powers JF, Sladek NE (1983) Cytotoxic activity relative to 4-Hydroxycyclophosphamide and phosphoramide mustard concentrations in the plasma of cyclophosphamide-treated rats. Cancer Res 43: 1101–1106
Alberts DS, Einspahr JG, Struck R, Bignami G, Young L, Surwit EA, Salmon SE (1984) Comparative in vitro cytotoxicity of cyclophosphamide, its major active metabolites and the new oxazaphosphorine ASTA Z 7557 (INN mafosfamide). Invest New Drugs 2: 141–148
Zaharko DS, Covey JM, Morpel G (1984) Observations on the effects of cyclophosphamide, phosphoramide mustard and some activated oxazaphosphorines on murine L1210 leukemia. Invest New Drugs 2: 149–154
Hohorst HJ, Draeger U, Peter G, Voelcker G (1976) The problem of oncostatic specificity of cyclophosphamide (NSC-26 271) studies on reactions that control the alkylating and cytotoxic activity. Cancer Treat Rep 60: 309–315
Friedman OM, Wodinsky I, Myles A (1976) Cyclophosphamide (NSC-26 271)-related phosphoramide mustards. Recent advances and historical perspectives. Cancer Treat Rep 60: 337–346
Domeyer BE, Sladek NE (1980) Kinetics of cyclophosphamide transformation in vivo. Cancer Res 40: 174–180
Sladek NE, Powers JF, Grage GM (1984) Half-life of oxazaphosphorines in biological fluids. Drug Metab Dispos 12: 553–559
Nathanson L, Hall TC, Rutenberg A, Shadduck RK (1967) Clinical toxicologic study of cyclohexylamine salt ofN,N-bis(2-chlorethyl)phosphorodiamidic acid (NSC-69 945; OMF-59). Cancer Chemother Rep 51: 35–39
Watson E (1987) Stability and disposition studies of cyclophosphamide metabolites. PhD thesis, University of South California at Los Angeles
Srigritsanapol A (1990) Kinetics of formation and disposition of 4-hydroxycyclophosphamide in the rat. Masters thesis. University of South California at Los Angeles
Cox PJ (1979) Cyclophosphamide cystitis-identification of acrolein as the causative agent. Biochem Pharmacol 28: 2045–2049
Cox PJ, Abel G (1979) Cyclophosphamide cystitis. 28: 3499–3502
Kwon CH, Maddison K, LoCastro L, Borch RF (1987) Accelerated decomposition of 4-hydroxycyclophosphamide by human serum albumin. Cancer Res 47: 1505–1508
Zheng JW, Chan KK, Ardalan B, Hengst J, Muggia FM (1992) One hour exposure is not adequate for cytotoxic evaluation of some alkylating agents. Proc Am Assoc Cancer Res 33: 403
Chan KK, Hong PS, Tutsch KK, Trump DL (1990) Metabolite profiles in patients receiving cyclophosphamide (CTX) alone and with SR2508. Proc Am Assoc Cancer Res 31: 183
Author information
Authors and Affiliations
Additional information
The work reported was supported by USC Cancer Center Core grant CA 14089 from the National Cancer Institute
Present address: Room 308, Comprehensive Cancer Center, The Ohio State University, 410 W. 12th Avenue, Columbus, Ohio 43210, USA
Rights and permissions
About this article
Cite this article
Zheng, J.J., Chan, K.K. & Muggia, F. Preclinical pharmacokinetics and stability of isophosphoramide mustard. Cancer Chemother. Pharmacol. 33, 391–398 (1994). https://doi.org/10.1007/BF00686268
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686268