Abstract
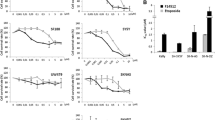
1,14-Bis-(ethyl)-amino-5,10-diazatetradecaneN 1,N 11-bis(ethyl)norspermine (BE-4-4-4) and 1,19-bis-(ethylamino)-5,10,15 triazanonadecane (BE-4-4-4-4) are two relatively new polyamine analogs synthesized for use as antineoplastic agents. In human brain tumor cell lines U-251 MG and SF-767, both agents inhibited cell growth, were cytotoxic, induced a variable G1/S block, and depleted intracellular polyamines. Since intracellular polyamine depletion did not always correlate with growth inhibition, cell survival, or cell cycle progression, it cannot completely explain the effects of these agents on growth, survival, and cell cycle progression in U-251 MG and SF-767 cells.
Similar content being viewed by others
References
Albanese L, Bergeron RJ, Pegg AE (1993) Investigations of the mechanism by which mammalian cell growth is inhibited by N1,N12-bis(ethyl)spermine. Biochem J 291:131
Barranco SC, Ford PJ, Townsend CM Jr (1986) Cell cycle kinetics responses of human stomach cancer cells to reduction in polyamine levels by treatment with alpha-difluoromethylornithine in vitro. Invest New Drugs 4:347
Basu HS, Feuerstein BG, Deen DF, Lubich WP, Bergeron RJ, Samejima K, Marton LJ (1989) Correlation between the effects of polyamine analogues on DNA conformation and cell growth. Cancer Res 49:5591
Basu HS, Pellarin M, Feuerstein BG, Deen DF, Bergeron RJ, Marton LJ (1990) Effect of N1,N14-bis(ethyl)homospermine on the growth of U-87 MG and SF-126 human brain tumor cells. Cancer Res 50:3137
Basu HS, Schwietert HCA, Feuerstein BG, Marton LJ (1990) Effects of variation in the structure of spermine on the association with DNA and the induction of DNA conformational changes. Biochem J 269:329
Basu HS, Pellarin M, Feuerstein BG, Deen DF, Marton LJ (1991) Effect of N1,N14-bis-(ethyl)-homospermine (BE-4-4-4) on the growth of U-251 MG and SF-188 human brain tumor cells. Int J Cancer 48:873
Basu HS, Sturkenboom MCJM, Delcros J-G, Csokan PP, Szollosi J, Feuerstein BG, Marton LJ (1992) Effect of polyamine depletion on chromatin structure in U-87 MG human brain tumour cells. Biochem J 282:723
Basu HS, Pellarin M, Feuerstein BG, Shirahata A, Samejima K, Deen DF, Marton LJ (1993) Interaction of a polyamine analogue, 1,19-bis-(ethylamino)-5,10,15-triazanonadecane (BE-4-4-4-4), with DNA and effect on growth, survival, and polyamine levels in seven human brain tumor cell lines. Cancer Res 53:3948
Bepler G, Carney DN, Nau MM, Gazdar AF, Minna JD (1986) Additive and differential biological activity of alpha-interferon A, difluoromethylornithine, and their combination on established human lung cancer cell lines. Cancer Res 46:3413
Bergeron RJ, Hawthorne TR, Vinson JRT, Beck DE Jr, Ingeno MJ (1989) Role of the methylene backbone in the antiproliferative activity of polyamine analogues on L1210 cells. Cancer Res 49:2959
Casero RA Jr, Ervin SJ, Celano P, Baylin SB, Bergeron RJ (1989) Differential response to treatment with the bis(ethyl)polyamine analogues between human small cell lung carcinoma and undifferentiated large cell lung carcinoma in culture. Cancer Res 49:639
Crissman HA, Mullaney PF, Steinkamp JA (1975) Methods and applications of flow systems for analysis and sorting of mammalian cells. Methods Cell Biol 9:179
Deen DF, Bartle PM, Williams ME (1979) Response of cultured 9L cells to spirohydantoin mustard and x-rays. Int J Radiat Oncol Biol Phys 15:1663
Dolan ME, Fleig MJ, Feuerstein BG, Basu HS, Luk GD, Casero RA Jr, Marton LJ (1994) Effect of 1,19-bis(ethylamino)-5,10,15-triazanonadecane on human tumor xenografts. Cancer Res 54:4698
Feuerstein BG, Williams LD, Basu HS, Marton LJ (1991) Implications and concepts of polyamine-nucleic acid interactions. J Cell Biochem 46:37
Fogel-Petrovic M, Shappell NW, Bergeron RJ, Porter CW (1993) Polyamine and polyamine analog regulation of spermidine/spermine N1-acetyltransferase in MALME-3M human melanoma cells. J Biol Chem 268:19118
Ghoda L, Basu HS, Porter CW, Marton LJ, Coffino P (1992) Role of ornithine decarboxylase suppression and polyamine depletion in the antiproliferative activity of polyamine analogs. Mol Pharmacol 42:302
Heby O, Jänne J (1981) Polyamine antimetabolites. Biochemistry, specificity, and biological effects of inhibitors of polyamine synthesis. In: Morris DR, Marton LJ (eds) Polyamines in biology and medicine. Marcel Dekker, New York, p 243
Hessels J, Kingma AW, Muskief FA, Sarhan S Seiler N (1991) Growth inhibition of two solid tumors in mice, caused by polyamine depletion, is not attended by alterations in cell-cycle phase distribution. Int J Cancer 48:697
Kabra PM, Lee HK, Lubich WP, Marton LJ (1986) Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr 380:19
Koza RA, Herbst EJ (1992) Deficiencies in DNA replication and cell cycle progression in polyamine-depleted HeLa cells. Biochem J 281:87
McCann PP, Pegg AE, Sjoerdsma A (1987) Inhibition of polyamine metabolism. Academic Press, Orlando
Oredsson SM, Deen DF, Marton LJ (1983) Influence of polyamine depletion caused by α-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase, on alkylation- and carbamoylation-induced cytotoxicity in 9L rat brain tumor cells in vitro. Cancer Res 43:4606
Pegg AE, Wechter R, Pakala R, Bergeron RJ (1989) Effect of N1,N12-bis(ethyl)spermine and related compounds on growth and polyamine acetylation, content, and excretion in human colon tumor cells. J Biol Chem 264:11744
Porter CW, Ganis B, Vinson T, Marton LJ, Kramer DL, Bergeron RJ (1986) Comparison and characterization of growth inhibition in L1210 cells by alpha-difluoromethylornithine, an inhibitor of ornithine decarboxylase, and N1,N8-bis(ethyl)spermidine, an apparent regulator of the enzyme. Cancer Res 46:6279
Porter CW, Pegg AE, Ganis B, Madhabala R, Bergeron RJ (1990) Combined regulation of ornithine and S-adenosylmethionine decarboxylases by spermine and the spermine analogue N1, N12-bis(ethyl)spermine. Biochem J 268:207
Porter CW, Ganis B, Libby PR, Bergeron RJ (1991) Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res 51:3715
Porter CW, Bernacki RJ, Miller J, Bergeron RJ (1993) Antitumor activity of N1,N11-bis(ethyl)norspermine against human melanoma xenografts and possible biochemical correlates of drug action. Cancer Res 53:581
Seidenfeld J, Gray JW, Marton LJ (1981) Depletion of 9L rat brain tumor cell polyamine content by treatment with DL-alpha-difluoromethylornithine inhibits proliferation and the G1 to S transition. Exp Cell Res 131:209
Seidenfeld J, Block AL, Komar KA, Naujokas MF (1986) Altered cell cycle phase distributions in cultured human carcinoma cells partially depleted of polyamines by treatment with difluoromethylornithine. Cancer Res 46:47
Sunkara PS, Fowler SK, Nishioka K (1981) Selective killing of transformed cells in combination with inhibitors of polyamine biosynthesis and S-phase specific drugs. Cell Biol Int Rep 5:991
Sunkara PS, Baylin SB, Luk GD (1987) Inhibitors of polyamine biosynthesis: cellular and in vivo effect on tumor proliferation. In: McCann PP, Pegg AE, Sjoerdsma A (eds) Inhibition of polyamine metabolism. Academic Press, Orlando, p 121
Author information
Authors and Affiliations
Additional information
This work was supported by grants CA 13525 and CA 49409 from the National Cancer Institute, by the Association pour la Recherche sur le Cancer (Villejuif), and by the National Brain Tumor Foundation
Rights and permissions
About this article
Cite this article
Bergeron, C.J., Basu, H.S., Marton, L.J. et al. Two polyamine analogs (BE-4-4-4 and BE-4-4-4-4) directly affect growth, survival, and cell cycle progression in two human brain tumor cell lines. Cancer Chemother. Pharmacol. 36, 411–417 (1995). https://doi.org/10.1007/BF00686190
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686190