Abstract
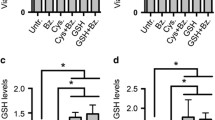
Levels of intracellular glutathione (GSH) and the GSH-related enzymes γ-glutamylcysteine synthetase (γ-GCS) and γ-glutamyltranspeptidase (γ-GT) were measured in the melphalan-resistant human multiple myeloma cell line 8226/LR-5 and were compared to those measured in the drug-sensitive 8226/S and doxorubicin-resistant 8226/Dox40 cell lines. Both GSH and γ-GCS activity, the rate-limiting step in the de novo synthesis of GSH, were elevated by a factor of approximately 2 in the melphalanresistant 8226/LR-5 cells relative to the other two lines. γ-GT activity was not elevated significantly in the /LR-5 cells. Northern analysis with a probe specific for the large subunit of human liver γ-GCS identified two bands (3.2 and 4.0 kb), both of which were increased by a factor of 2–3 in the 8226/LR-5 line. Levels of γ-GCS mRNA expression were comparable in the /S and /Dox40 cell lines. Levels of γ-GT mRNA were similar in the /S and /LR-5 lines but were reduced in the /Dox40 cells. These data suggest that the increased GSH levels associated with resistance to melphalan in the 8226/LR-5 myeloma cells is attributable to up-regulation of γ-GCS. This observation is consistent with recent demonstrations of up-regulation of γ-GCS in melphalan-resistant prostate carcinoma cells and cisplatinum-resistant ovarian carcinoma cells, suggesting that increased expression of γ-GCS may be an important mediator of GSH-associated resistance mechanisms.
Similar content being viewed by others
References
Ahmad S, Okine L, Le B, Najarian P, Vistica DT (1987) Elevation of glutathione in phenylalanine mustard-resistant murine L1210 leukemia cells. J Biol Chem 262:15048
Ahmad S, Okine L, Wood R, Aljian J, Vistica DT (1987) γ-Glutamyl transpeptidase (γ-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J Cell Physiol 131:240
Bailey HH, Gipp JJ, Ripple M, Wilding G, Mulcahy RT (1992) Increase in γ-glutamylcysteine synthetase activity and steady-state messenger RNA levels in melphalan-resistant DU-145 human prostate carcinoma cells expressing elevated glutathione levels. Cancer Res 52:5115
Bailey HH, Mulcahy RT, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M, Spriggs DR (1993) A phase I clinical trial of intravenous l-buthionine sulfoximine (NSC-326331) and melphalan: an attempt at modulation of glutathione. J Clin Oncol (in press)
Bellamy WT, Dalton WS, Meltzer, Dorr RT (1989) Role of glutathione and its associated enzymes in multidrug-resistant human myeloma cells. Biochem Pharmacol 38:787
Bellamy WT, Dalton WS, Gleason MC, Grogan TM, Trent JM (1991) Development and characterization of a melphalan-resistant human multiple myeloma cell line. Cancer Res 51:995
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248
Bump EA, Taylor YC, Brown JM (1983) Role of glutathione in the hypoxic cell cytotoxicity of misonidazole. Cancer Res 43:997
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156
Chung AS, Maines MD (1981) Effect of selenium on glutathione metabolism. Induction of γ-glutamylcysteine synthetase and and glutathione reductase in the rat liver. Biochem Pharmacol 30:3217–3223
Gipp JJ, Chang C, Mulcahy RT (1992) Cloning and nucleotide sequence of a full-length cDNA for human liver γ-glutamylcysteine synthetase. Biochem Biophys Res Commun 185:29
Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME (1992) High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA 89:3070
Goodspeed DC, Dunn TJ, Miller CD, Pitot HC (1989) Human γ-glutamyl transpeptidase cDNA: comparison of hepatoma and kidney mRNA in the human and rat. Gene 76:1
Griffith OW (1982) Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitiors of glutathione synthesis. J Biol Chem 257:131704
Griffith OW, Meister A (1979) Glutathione: interorgan translocation, turnover and metabolism. Proc Natl Acad Sci USA 76:5606
Hamilton TC, Winker MA, Louis KG, et al (1985) Augmentation of Adriamycin, melphalan and cisplatin cytotoxicity in drug-resistant and-sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol 34:2583
Kramer RA, Greene K, Ahmad S, et al (1987) Chemosensitization of 1-phenylalanine mustard by the thiol-modulating agent buthionine sulfoximine. Cancer Res 47:1593
Lewis AD, Hayes JD, Wolf CR (1988) Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis 9:1283
Meister A (1989) Metabolism and function of glutathione. In: Dolphin D, Poulson R, Avramovic O (eds) Coenzymes and cofactors. Glutathione: chemical, biochemical and medical aspects. John Wiley and Sons, New York, p 367
Meister A, Tate SS, Griffith OW (1981) γ-Glutamyl transpeptidase. Methods Enzymol 77:237
Nardi G, Cipollaro M, Loguercio C (1990) Assay of γ-glutamylcysteine synthetase and glutathione synthetase in erythrocytes by high-performance liquid chromatography with fluorimetric detection. J Chromatogr 530:122
O'Dwyer PJ, Hamilton TC, Young RC, LaCreta FP, Carp N, Tew KD, Padavic K, Comis RL, Ozols RF (1992) Depletion of glutathione in normal and malignant human cells in vivo by buthionine sulfoximine: clinical and biochemical results. J Natl Cancer Inst 84:264
Ogino T, Kawabata T, Awai M (1989) Stimulation of glutathione synthesis in iron-loaded mice. Biochim Biophys Acta 1006:131
Ono K, Komuro C, Tsutsui K, Shibamoto Y, Nishidai T, Takahashi M, Abe M, Shrieve DC (1986) Combined effect of buthionine sulfoximine and cyclophosphamide upon murine tumours and bone marrow. Br J Cancer 54:749
Russo A, Carmichael J, Friedman N, DeGraff W, Tochner Z, Glatstein E, Mitchell JB (1986) The roles of intracellular glutathione in antineoplastic chemotherapy. Int J Radiat Oncol Biol Phys 12:1347
Tietze F (1969) Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Application to mammalian blood and other tissues. Anal Biochem 27:502
Wolf CR, Lewis AD, Carmichael J, Adams DJ, Allan SG, Ansell DJ (1987) The role of glutathione in determining the response of normal and tumour cells to anticancer drugs. Biochem Soc Trans 15:728
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mulcahy, R.T., Bailey, H.H. & Gipp, J.J. Up-regulation of γ-glutamylcysteine synthetase activity in melphalan-resistant human multiple myeloma cells expressing increased glutathione levels. Cancer Chemother. Pharmacol. 34, 67–71 (1994). https://doi.org/10.1007/BF00686114
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686114