Abstract
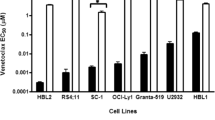
A series of five potential modulators of resistance were tested for their relative ability, as compared with verapamil, to sensitize CEM lymphoblastoid leukemia drug-resistant tumor sublines expressing either the classic or the atypical multidrug-resistance (MDR) phenotype to vinblastine or teniposide. Maximal non-cytotoxic concentrations of each modulator were tested and sensitization indices (SIs) were derived by comparing the drug concentration required to inhibit growth by 50% in their presence or absence. Like verapamil (10 μM) itself, three of the other modulators tested, namely, S9788 (4 μM), flunarizine (20 μM) and quinidine (30 μM), resulted in 2-to 3-fold sensitization of vinblastine against the parental CEM cells, and comparable effects were noted in the CEM/VM-1 cells, which were not cross-resistant to vinblastine. In contrast, cyclosporin A (0.5 μM) and B859-35 (2 μM) did not enhance vinblastine growth inhibition in these lines. However, the greatest sensitization with all the modulators was noted in the classic MDR VBL 1000 cells, with SIs ranging from 40- to 350-fold, except for cyclosporin A, which proved ineffective at the concentration tested (SI, 2.6). The greatest extent of differential sensitization of these VBL1000 tumor cells occurred with quinidine or B859-35, which proved significantly more effective than verapamil alone. Combinations of modulators resulted in additive effects, with B859-35 plus cyclosporin A proving superior to B859-35 plus verapamil. In contrast, none of these compounds proved effective as a sensitizer to teniposide. The growth-inhibitory effects of this drug were not modified significantly in either the 92-fold teniposideresistant VM-1 cells or in the parental cells. Addition of verapamil itself also failed to modulate teniposide growth inhibition in the VBL1000 cells, which express significant cross-resistance to this drug (36-fold). However, SI values of 3- to 5-fold were obtained using quinidine or B859-35. These results serve (a) to emphasise the need to monitor the effects of modulators not only on drug-resistant cells but also on their drug-sensitive counterparts so as to ensure differential sensitization such that normal sensitive tissues are not likely to be adversely influenced and (b) to highlight the observation that the extent of modulation differs depending not only on the antitumor drug used but also on the mechanism of drug resistance expressed. This in vitro model system appears to provide a useful screening system for resistance modulators and certainly could be used in attempts to identify alternative agents that may influence teniposide sensitivity in these drug-resistant sublines.
Similar content being viewed by others
References
Akiyama SI, Cornwell MM, Kuwano M, Pastan I, Gottesman MM (1988) Most drugs that reverse multidrug resistance also inhibit photoaffinity labelling of P-glycoprotein by a vinblastine analog. Mol Pharmacol 33: 144–147
Barrand MA, Rhodes T, Center MS, Twentyman PR (1993) Chemosensitisation and drug accumulation effects of cyclosporin A, PSC-833 and verapamil in human MDR large cell lung cancer cells expressing a 190k membrane protein distinct from P-glycoprotein. Eur J Cancer 29 A: 408–415
Beck WT (1991) Modulators of B-glycoprotein-associated multidrug resistance. In: Ozols RF (eds) Molecular and clinical advances in anticancer drug resistance. Kluwer, Boston, pp 151–170
Beck WT, Cirtain MC, Danks MK, Felsted RL, Safa AR, Wolverton JS, Suttle DP, Trent JM (1987) Pharmacological, molecular, and cytogenetic analysis of “atypical” multidrug-resistant human leukemic cells. Cancer Res 47: 5455–5460
Beck WT, Cirtain MC, Look AT, Ashmun RA (1986) Reversal of vinca alkaloid resistance but not multiple drug resistance in human leukemic cells by verapamil. Cancer Res 46: 778–784
Bellamy WT, Dalton WS, Kailey JM, Gleason MC, McCloskey TM, Door RT, Alberts DS (1988) Verapamil reversal of doxorubicin resistance in multidrug-resistant human myeloma cells and association with drug accumulation and DNA damage. Cancer Res 48: 6303–6308
Boesch D, Gaveriaux C, Jachez B, Pourtier-Manzanedo A, Bollinger P, Loor F (1991) In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res 51: 4226–4233
Bradley G, Juranka PF, Ling V (1988) Mechanisms of multidrug resistance. Biochim Biophys Acta 948: 87–128
Chambers SK, Hait WN, Kacinski BM, Keyes SR (1989) Enhacement of anthracycline growth inhibition in parent and multidrug-resistant Chinese hamster ovary cells by cyclosporin A and its analogues. Cancer Res 49: 6725–6729
Cole SPC, Downes HF, Slovak ML (1989) Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer 59: 42–46
Coon JS, Knudson W, Clodfelter K, Lu B, Weinstein RS (1991) Solutol HS 15, nontoxic polyoxyethylene esters of 12-hydroxystearic acid, reverses multidrug resistance. Cancer Res 51: 897–902
Danks MK, Schmidt CA, Cirtain MC, Suttle DP, Beck WT (1988) Altered catalytic activity of and DNA cleavage by DNA topoisemcrase II from human leukemic cells selected for resistance to VM-26. Biochemistry 27: 8861–8869
Danks MK, Yalowich JC, Beck WT (1987) Atypical multiple drug resistance in a human leukemic cell line selected for resistance to teniposide. Cancer Res 47: 1297–1301
Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE (1965) Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer 18: 522–529
Ford JM, Hait WN (1990) Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev 42: 155–159
Georges E, Sharom FJ, Ling V (1990) Multidrug resistance and chemosensitization: therapeutic implications for cancer chemotherapy. Adv Pharmacol 21: 185–220
Gerlach JH, Bell DR, Karakousis C, Slocum HK, Kartner N, Rustum YM, Ling V, Baker RM (1987) P-glycoprotein in human sarcoma: evidence for multidrug resistance. J Clin Oncol 5: 1452–1460
Goldstein LJ, Galski H, Fojo A, Willingham M, Lai S-L, Gazdar A, Pirker R, Green JA, Crist W, Brodeur GM, Lieder M, Cossman J, Gottesman MM, Pastan I (1989) Expression of multidrug resistance gene in human cancers. J Natl Cancer Inst 81: 116–124
Gottesman MM, Pastan I (1989) Clinical trials of agents that reverse multidrug resistance. J Clin Oncol 7: 409–410
Hill BT, Graaf WTA van der, Hosking LK, Vries EGE de, Mulder NH, Whelan RDH (1993) Evaluation of S9788 as a potential modulator of drug resistance against human tumour sublines expressing differing resistance mechanisms in vitro. Int J Cancer (in press)
Hofmann J, Ueberall F, Egle A, Grunicke H (1991) B-859-35, a new drug with anti-tumor activity reverses multi-drug resistance. Int J Cancer 47: 870–874
Holzmayer TA, Hilsenbeck S, Von Hoff DD, Roninson IB (1992) Clinical correlates ofmdr 1 (P-glycoprotein) gene expression in ovarian and small-cell lung carcinomas. J Natl Cancer Inst 84: 1486–1491
Hosking LK, Hendriks H, Hill BT (1992) In vitro evaluation of the enhancement of vinblastine cytotoxicity using a panel of novel modulators. Br J Cancer 65 (Suppl XVI): 21
Hu XF, Martin TJ, Bell DR, Luise M de, Zalcberg JR (1990) Combined use of cyclosporin A and verapamil in modulating multidrug resistance in human leukemia cell lines. Cancer Res 50: 2953–2957
Ishida Y, Shimada Y, Shimoyama M (1990) Synergistic effect of cyclosporin A and verapamil in overcoming vincristine resistance of multidrug-resistant cultured human leukemia cells. Jpn J Cancer Res 81: 834–841
Kartner N, Everden-Porelle D, Bradley G, Ling V (1985) Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature 316: 820–823
Kavallaris M, Madafiglio J, Norris MD, Haber M (1993) Resistance to tetracycline, a hydrophilic antibiotic, is mediated by P-glycoprotein in human multidrug-resistant cells. Biochem Biophys Res Commun 190: 79–85
Klohs WD, Steinkampf RW, Havlick MJ, Jackson RC (1986) Resistance to anthrapyrazoles and anthracyclines in multidrug resistant P388 murine leukemia cells: reversal by calcium blockers and calmodulin antagonists. Cancer Res 46: 4352–4356
Larsson R, Nygren P (1990) Pharmacological modification of multidrug resistance (MDR) in vitro detected by a novel fluorometric microculture cytotoxicity assay. Reversal of resistance and selective cytotoxic actions of cyclosporin A and verapamil on MDR leukemia T-cells. Int J Cancer 46: 67–72
Lehnert M, Dalton WS, Roe D, Emerson S, Salmon SE (1991) Synergistic inhibition by verapamil and quinine of P-glycoprotein-mediated multidrug resistance in a human myeloma cell line model. Blood 77: 348–354
Meijer C, Mulder NH, Timmer-Bosscha H, Peters WHM, Vries EGE de (1991) Combined in vitro modulation of Adriamycin resistance. Int J Cancer 49: 582–586
Miller TP, Grogan TM, Dalton WS, Spier CM, Scheper RJ, Salmon SE (1991) P-glycoprotein expression in malignant lymphoma and reversal of clinical drug resistance with chemotherapy plus high-dose verapamil. J Clin Oncol 9: 17–24
Naito M, Tsuge H, Kuroko C, Koyama T, Tomida A, Tatsuta T, Heike Y, Tsuruo T (1993) Enhancement of cellular accumulation of cyclosporine by anti-P-glycoprotein monoclonal antibody MRK-16 and synergistic modulation of multidrug resistance. J Natl Cancer Inst 85: 311–316
Nygren P, Larsson R (1991) Differential in vitro sensitivity of human tumor and normal cells to chemotherapeutic agents and resistance modulators. Ann Oncol 2: 305–306
Osann K, Sweet P, Slater LM (1992) Synergistic interaction of cyclosporin A and verapamil on vincristine and daunorubicin resistance in multidrug-resistant human leukemia cells in vitro. Cancer Chemother Pharmacol 30: 152–154
Pennock GD, Dalton WS, Roeske WR, Appleton CP, Mosley K, Plezia P, Miller TP, Salmon SE (1991) Systemic toxic effects associated with high-dose verapamil infusion and chemotherapy administration. J Natl Cancer Inst 83: 105–110
Pierré A, Dunn TA, Kraus-Berthier L, Léonce S, Saint-Dizier D, Régnier G, Dhainaut A, Berlion M, Bizzari J-P, Atassi G (1992) In vitro and in vivo circumvention of multidrug resistance by Servier 9788, a novel triazinoaminopiperidine derivative. Invest N Drugs 10: 137–148
Ramu A, Glaubiger D, Fuks Z (1984) Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analognes. Cancer Res 44: 4392–4395
Ramu A, Ramu N (1992) Reversal of multidrug resistance by phenothiazines and structurally related compounds. Cancer Chemother Pharmacol 30: 165–173
Safa Ar (1993) Photoaffinity labeling of P-glycoprotein in multidrug-resistant cells. Cancer Invest 11: 46–56
Salmon SE, Dalton WS, Grogan TM, Plezia P, Lehnert M, Roe DJ, Miller TP (1991) Multidrug-resistant myeloma: laboratory and clinical effects of verapamil as a chemosensitizer. Blood 78: 44–50
Sehested M, Friche E, Jensen PB, Demant EJF (1992) Relationship of VP-16 to the classical multidrug resistance phenotype. Cancer Res 52: 2874–2879
Slater LM, Murray SL, Wetzel MW, Sweet P, Stupecky M (1986) Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J Clin Invest 77: 1405–1408
Slovak ML, Hoeltge GA, Dalton WS, Trent JM (1988) Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res 48: 2793–2797
Sonneveld P, Durie BGM, Lokhorst HM, Marie J-P, Solbu G, Suciu S, Zittoun R, Lowenberg B, Nooter K (1992) Modulation of multidrug-resistant multiple myeloma by cyclosporin. Lancet 340: 255–259
Stallard S, Kaye SB (1989) Reversal of resistance in the breast cancer cell line MCF-7 ADR was most effective with the modulating agent quinidine. Br J Cancer 60: 500
Tsuruo T (1991) Reversal of multidrug resistance by calcium channel blockers and other agents. In: Roninson IB (ed) Molecular and cellular biology of multidrug resistance in tumor cells. Plenum, New York, pp 349–372
Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y (1981) Overcoming of vincristine resistance in P388 leukemia in vivo and intro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res 41: 1967–1972
Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y (1982) Increased accumulation of vincristine and Adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res 42: 4730–4733
Tsuruo T, Iida H, Kitatani Y, Yokota K, Tsukagoshi S, Sakurai Y (1984) Effects of quinidine and related compounds on cytotoxicity and cellular accumulation of vincristine and Adriamycin in drug-resistant tumor cells. Cancer Res 44: 4303–4307
Twentyman P, Fox N, Bleehan N (1987) Drug resistance in human lung-cancer cell lines: cross-resistance studies and effects of the calcium channel transport blocker, verapamil. Int J Radiat Oncol Biol Phys 12: 1355–1360
Twentyman PR (1992) Cyclosporins as drug resistance modifiers. Biochem Pharmacol 43: 109–117
Twentyman PR, Reeve JG, Koch G, Wright KA (1990) Chemosensitisation by verapamil and cyclosporin A in mouse tumour cells expressing different levels of P-glycoprotein and CP22 (sorein). Br J Cancer 62: 89–95
Bliek AM van der, Borst P (1989) Multidrug resistance. Adv Cancer Res 52: 165–203
Graaf WTA van der, Vries EGE de, Uges DRA, Nanninga AG, Vellenga E, Mulder POM, Mulder NH (1991) In vitro and in vivo modulation of multi-drug resistance with amiodarone. Int J Cancer 48: 616–622
Warr JR, Brewer F, Anderson M, Fergusson J (1986) Verapamil hypersensitivity of vincristine-resistant Chinese ovary cell lines. Cell Biol Int Rep 10: 389–399
Willingham MC, Cornwell MM, Cardarelli CO, Gottesman MM, Pastan I (1986) Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and-sensitive KB cells: effects of verapamil and other drugs. Cancer Res 46: 5941–5946
Wishart GC, Plumb JA, Morrison JG, Hamilton TG, Kaye SB (1992) Adequate tumour quinidine levels for multidrug resistance modulation can be achieved in vivo. Eur J Cancer 28: 28–31
Yang C-PH, Greenberger LM, Horwitz SB (1991) Reversal of multi-drug resistance in tumor cells. In: Chou T-C, Rideout DC (eds) Synergism and antagonism in chemotherapy. Academic Press, London, pp 311–338
Yang CH, DePinho SG, Greenberger LM, Arceci RH, Horwitz SB (1989) Progesterone interacts with P-glycoprotein in multidrug-resistant cells and in the endometrium of gravid uterus. J Biol Chem 264: 782–788
Zamora JM, Beck WT (1986) Chloroquine enhancement of anticancer drug cytotoxicity in multiple drug resistant human leukemic cells. Biochem Pharmacol 35: 4303–4310
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hill, B.T., Hosking, L.K. Differential effectiveness of a range of novel drug-resistance modulators, relative to verapamil, in influencing vinblastine or teniposide cytotoxicity in human lymphoblastoid CCRF-CEM sublines expressing classic or atypical multidrug resistance. Cancer Chemother. Pharmacol. 33, 317–324 (1994). https://doi.org/10.1007/BF00685907
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685907