Summary
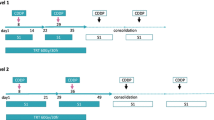
To investigate chemotherapeutic dose intensity in advanced non-small-cell lung cancer (NSCLC), we evaluated a pharmacokinetically designed schedule of high-dose cisplatin (200 mg/m2 per 28-day cycle) plus mitomycin C. Between March 1987 and March 1989, 62 patients were registered for a phase II study of the Northern California Oncology Group (NCOG). The treatment schedule consisted of cisplatin in hypertonic saline given on a divided days 1 and 8 schedule (100 mg/m2 on each day) plus mitomycin C given at a dose of 8 mg/m2 on day 1 of each cycle. In 61 patients evaluable for response analysis, the overall response rate was 39% (24/61), with a complete response being achieved in 6% (4/61) of cases and a partial response, in 33% (20/61). The response according to reviewed histologic subtype included squamous, 53% of patients (10/19); large cell, 31% (4/13); and adenocarcinoma, 34% (10/29). The median survival for all patients was 19.3 weeks. The mean cisplatin and mitomycin C delivered dose intensities in this study were 45 mg/m2 per week (90% of the projected dose) and 1.5 mg/m2 per week (75%). The toxicity of this combination regimen in the 62 enrolled patients was significant but manageable. Leukopenia (WBC, <1,000/mm3) and thrombocytopenia (platelets, <25,000/mm3) occurred in 3# and 8% of patients treated, respectively. Dose-limiting renal toxicity and clinically significant ototoxicity developed in 8 patients each (13%), and a peripheral sensory neuropathy was observed in 17 cases (27%). Whether this type of dose-intensive therapy results in an improved therapeutic index in NSCLC is currently being evaluated in a randomized comparative trial versus standard-dose cisplatin therapy.
Similar content being viewed by others
References
Armitage P (1971) Statistical methods in medical research. Blockwell, New York, p 131
Bhuchar VK, Lanzotti VJ (1982) High dose cisplatin for lung cancer. Cancer Treat Rep 66:375–376
Bonomi PD, Finkelstein DM, Ruckdeschel JC, Blum RH, Green MD, Mason B, Hahn R, Tormey DC, Harris J, Comis R, Glick J (1989) Combination chemotherapy versus single agents followed by combination chemotherapy in stage IV non-small-cell lung cancer: a study of the Eastern Cooperative Oncology Group. J Clin Oncol 7: 1602–1613
Britell JC, Eagan RT, Ingle JN, Creagan ET, Rubin J, Frytak S (1978)Cis-Dichlorodiammineplatinum(II) alone followed by Adriamycin plus cyclophosphamide at progression versuscis-dichlorodiammineplatinum(II), Adriamycin, and cyclophosphamide in combination for adenocarcinoma of the lung. Cancer Treat Rep 62:1207–1210
DeJager R, Longeval E, Klastersky J (1980) High-dose cisplatin with fluid and mannitol-induced diuresis in advanced lung cancer: a phase II clinical trial of the EORTC Lung Cancer Working Party (Belgium). Cancer Treat Rep 64:1341–1346
Gandara DR, Perez EA, Phillips WA, Lawrence HJ, DeGregorio MW (1989) Evaluation of cisplatin dose intensity: current status and future prospects. Anticancer Res 9:1121–1128
Gandara DR, DeGregorio MW, Wold HG, Perez EA, Deisseroth AB, Doroshow J, Meyers F, McWhirter K, Hannigan J (1989) Cisplatin dose intensity in non-small-cell lung cancer: phase II results of aday 1 and day 8 high-dose regimen. J Natl Cancer Inst 81:790–794
Gandara DR, DeGregorio MW, Wold HG, Wilbur BJ, Kohler M, Lawrence HJ, Deisseroth AB, George CB (1989) High-dose cisplatin in hypertonic saline: reduced toxicity of a modified dose schedule and correlation with plasma pharmacokinetics. A Northern California Oncology Group pilot study in non-small-cell lung cancer. J Clin Oncol 4:1787–1793
Gandara DR, Perez EA, Lawrence HJ, DeGregorio MW (1989) Phase I trial of high dose cisplatin plus diethyldithiocarbamate rescue: toxicity profile compared to patients receiving high dose cisplatin alone. Proc Am Assoc Cancer Res 30:241
Hoffman PC, Bitran JD, Golomb HM (1983) Chemotherapy of metastatic non-small-cell bronchogenic carcinoma. Semin Oncol 10(1):111–122
Hryniuk W, Bush H (1984) The importance of dose intensity of chemotherapy of metastatic breast cancer. J Clin Oncol 2:1281–1288
Hutchison FN, Perez EA, Gandara DR, Lawrence HJ, Kaysen GA (1988) Renal salt wasting in patients treated with cisplatin. Ann Intern Med 108:21–25
Kaplan EL, Meyer P (1958) Nonparametric estimation from imcomplete observations. J Am Stat Assoc 53:457–481
Klastersky JP, Schulier G, Bureau P, Libert P, Ravez P, Vandermoten G, Thiriaux J, Lecomte J, Cordier R, Dabouis G, Brohee D, Themelin L, Mommen P (1989) Cisplatin versus cisplatin plus etoposide in the treatment of advanced non-small-cell lung cancer. J Clin Oncol 7:1087–1092
Kris MG, Gralla RJ, Wertheim MS, Kelsen DP, O'Connell JP, Burke MT, Fiore JJ, Cibas IR, Heelan RT (1986) Trial of the combination of mitomycin, vindesine, and cisplatin in patients with advanced non-small-cell lung cancer. Cancer Treat Rep 70(9):1091–1096
Mountain CF (1986) A new international staging system for lung cancer. Chest 89:225S-233S
Ozols RF, Corden BJ, Jacob J, Wesley MN, Dstellega Y, Young RC (1984) High-dose cisplatin in hypertonic saline. Ann Intern Med 100:19–26
Ozols RF, Ostchega Y, Myers CE, Young RC (1985) High-dose cisplatin in hypertonic saline in refractory ovarian cancer. J Clin Oncol 3:1246–1250
Panettiere FJ, Vance RB, Stuckey WJ (1983) Evaluation of single-agent cisplatin in the management of non-small-cell carcinoma of the lung: a Southwest Oncology Group study. Cancer Treat Rep 67:399–400
Perez EA, Putney JD, Gandara DR (1989) In vitro dose-response relationship to cisplatin in human non-small-cell lung cancer cell lines. Proc Am Assoc Cancer Res 30:459
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Petro J, Smith PG (1977) Design and analysis of randomized clinical trials requiring prolonged observation of each patients. Br J Cancer 35:1–39
Rapp EA, Pater JL, Willan A, Cormier Y, Murray N, Evans WK, Hodson DI, Clark DA, Feld R, Arnold AM, Ayoub JI, Wilson KS, Latreille J, Wierzbicki RF, Hill DP (1988) Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer —report of a Canadian multicenter randomized trial. J Clin Oncol 6: 633–641
Sadler JG, Natale RB (1988) Basis for experimental chemotherapy in lung cancer. Semin Oncol 15:32–36
Sculier JP, Klastersky J (1984) Progress in chemotherapy of non-small-cell lung cancer. Eur J Cancer Clin Oncol 20:1329–1333
Vogl SE, Berenzweig M, Camacho F, Greenwald E, Kaplan BH (1982) Efficacy study of intensive cisplatin therapy in advanced non-small-cell bronchogenic carcinoma. Cancer 50:24–26
Author information
Authors and Affiliations
Additional information
This research was supported in part by grants CA 21 744, CA 25 838, CA 25 827, CA 45 466, CA 37 457, CA 39 098, CA 35 016, CA 35 283, Bristol-Myers Company, and the Veterans Administration. One of the authors (R.W.C.) is the recipient of an American Cancer Society Clinical Oncology Cancer Development Award
Rights and permissions
About this article
Cite this article
Gandara, D.R., Perez, E.A., Wold, H. et al. High-dose cisplatin and mitomycin C in advanced non-small cell lung cancer: a phase II study of the Northern California Oncology Group. Cancer Chemother. Pharmacol. 27, 243–247 (1990). https://doi.org/10.1007/BF00685721
Issue Date:
DOI: https://doi.org/10.1007/BF00685721