Summary
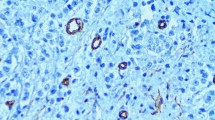
In the present study we update previous results on the prognostic value of intratumoral microvessel density (IMD), determined immunocytochemically using the monoclonal antibody CD-31 and a standard streptavidin-immunoperoxidase technique, published in theJ Clin Oncol 12:454–466, 1994. This study was undertaken in those 211 node-negative breast cancer (NNBC) cases of that series of which we had pathological material available to determine all the prognostic indicators. The median period of follow-up has been extended to 78 and 80 months for relapse-free survival (RFS) and overall survival (OS), respectively, and new biological indicators (i.e. Ki-67 labeling and 67 kDa laminin receptor expression) were included in the analysis.
The main results obtained are:i) a confirmation that IMD is not associated with the other biological markers studied, i.e. expression of p53 protein, c-erbB-2 protein, 67 kDa laminin receptor, and cell kinetics; IMD was weakly associated only with histological grade (p=0.053);ii) IMD remains a highly significant prognostic factor for RFS and OS (p<0.0001 and p=0.018, respectively) in univariate analysis;iii) in multivariate analysis on RFS, IMD (likelihood ratio test (LRT)=30.16; p<0.0001), 67 kDa laminin receptor (LRT=9.80; p=0.0017), the IMD/67 kDa laminin receptor interaction (LRT=8.62; p=0.0033), tumor size (LRT=8.56; p=0.0034), and p53 protein (LRT=4.96; p=0.025) are significant and independent prognostic indicators. For OS, only tumor size (LRT=8.34; p=0.0038), menopausal status (LRT=5.16; p=0.023), p53 protein (LRT=4.37; p=0.036), and IMD (LRT=4.05; p=0.044) retain a significant and independent prognostic value.
The results of this study confirm the prognostic importance on RFS of the variables previously tested, but not of peritumoral lymphatic vessel invasion. A novel finding is that 67 kDa laminin receptor and the IMD/67 kDa laminin receptor interaction are also significant and independent variables. For OS, the results confirm that both IMD and tumor size are significant and independent variables. With prolonged follow-up the novel finding that emerges is the prognostic importance of menopausal status and p53 protein.
This new information could be useful for a more accurate selection of high-risk NNBC patients who require careful follow-up and may benefit from adjuvant therapy.
Similar content being viewed by others
References
Folkman J: How is blood vessel growth regulated in normal and neoplastic tissue? Cancer Res 46:467–475, 1986.
Folkman J: Introduction: Angiogenesis and cancer. Sem Cancer Biol 3:47–48, 1992.
Liotta L, Kleinerman J, Saldel G: Quantitative relationship of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res 34:997–1004, 1974.
Dameron KM, Volpert OV, Tainsky MA, Bouck N: Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 265:1582–1585, 1994.
Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A: Glioblastoma growth inhibitedin vivo by a dominant-negative F1K-1 mutant. Nature 367:576–579, 1994.
Kandel J, Bossy-Wetzel E, Radvanyi F, Klagsbrun M, Folkman J, Hanahan D: Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell 66:1095–1104, 1991.
D'Amore PA: Capillary growth: a two-cell system [review]. Sem Cancer Biol 3:49–56, 1992.
Leibovich SJ, Polverini PJ, Shepard HM: Macrophage-induced angiogenesis mediated by tumour necrosis factor-alpha. Nature 329:630–634, 1987.
Starkey JR, Crowle PK, Taubenberger S: Mast-cell-deficient W/Wv mice exhibit a decrease rate of tumor angiogenesis. Int J Cancer 42:48–52, 1988.
Goto F, Goto K, Weindel F, Folkman J: Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor in the bovine capillary endothelial cells within collagen gels. Lab Invest 69:508–517, 1993.
Brooks PC, Clark RAF, Cheresh DA: Requirement of vascular integrin αVβ3 for angiogenesis. Science 264:569–571, 1994.
Rastinejad F, Polverini PJ, Bouk NP: Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell 56:345–355, 1989.
Kieser A, Weich HA, Brandner G, Marmè D, Kolch W: Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene 9:963–969, 1994.
Bouck NP: Tumor angiogenesis: The role of oncogenes and tumor suppressor genes. Cancer Cell 2:179–185, 1990.
Rak JW, Hegman E, Lu C, Kerbel RS: Progressive loss of sensitivity to endothelium-derived growth inhibitors expressed by human melanoma cells during disease progression. J Cell Physiol 159:245–255, 1994.
Folkman J, Watson K, Ingber D, Hanahan D: Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339:58–61, 1989.
Fidler IJ, Ellis LM: The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 79:185–188, 1994.
O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J: Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79:315–328, 1994.
Gasparini G, Harris AL: Clinical importance of the determination of tumor angiogenesis in breast carcinoma: Much more than a new prognostic tool [review]. J Clin Oncol 13:765–782, 1995.
Craft PS, Harris AL: Clinical prognostic significance of tumour angiogenesis [review]. Ann Oncol 5:305–311, 1994.
Gasparini G: Quantification of intratumoral vascularisation predicts metastasis in human invasive solid tumours [review]. Oncol Rep 1:7–12, 1994.
Gasparini G, Weidner N, Bevilacqua P, Maluta S, Dalla Palma P, Caffo O, Barbareschi M, Boracchi P, Marubini E, Pozza F: Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma. J Clin Oncol 12:454–466, 1994.
Weidner N, Semple SP, Welch WR, Folkman J: Tumor angiogenesis and metastasis — correlation in invasive breast carcinoma. N Engl J Med 324:1–8, 1991.
Wright C, Angus B, Nicholson S, Sainsbury JRC, Cairns J, Gullick WJ, Kelly P, Harris AL, Horne CHW: Expression of c-erbB-2 oncoprotein: A prognostic indicator in human breast cancer. Cancer Res 49:2087–2090, 1989.
Martignone S, Pellegrini R, Villa E, Tandon NN, Mastroianni A, Tagliabue E, Ménard S, Colnaghi MI: Characterization of two monoclonal antibodies directed against the 67KDa high affinity laminin receptor and application for the study of breast carcinoma progression. Clin Exp Metast 10:379–386, 1992.
Martignone S, Ménard S, Bufalino R, Cascinelli N, Pellegrini R, Tagliabue E, Andreola S, Rilke F, Colnaghi MI: Prognostic significance of the 67-kilodalton LRec expression in human breast carcinomas. J Natl Cancer Inst 85:398–402, 1993.
Gerdes J, Schwab U, Lamke H, Stein H: Production of mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20, 1983.
Gasparini G, Pozza F, Bevilacqua P, Meli S, Boracchi P, Reitano M, Santini G, Marubini E, Sainsbury JRC: Growth fraction (Ki-67 antibody) determination in early-stage breast carcinoma: Histologic, clinical, and prognostic correlations. The Breast 1:92–99, 1992.
Gore SM, Pocock SJ, Kerr GR: Regression models and non-proportional hazards in the analysis of breast cancer survival. Appl Statist 33:176–195, 1984.
Andersen PK, Borgan O, Dill RD, Keiding N (eds): Statistical Models Based on Counting Processes. Springer-Verlag, New York, 1993.
McGuire WL: Breast cancer prognostic factors: Evaluation guidelines [editorial]. J Natl Cancer Inst 83:154–155, 1991.
Gimbrone MA Jr, Gullino PM: Neovascularization induced by intraocular xenografts of normal, preneoplastic, and neoplastic mouse mammary tissues. J Natl Cancer Inst 56:305–318, 1976.
Brem SS, Jensen HM, Gullino PM: Angiogenesis as a marker of preneoplastic lesions of the human breast. Cancer 41:239–244, 1978.
Jensen HM, Chen I, DeVault MR, Lewis AE: Angiogenesis induced by “normal” human breast tissue: A probable marker for precancer. Science 218:293–295, 1982.
Guidi AJ, Fischer L, Harris JR, Schnitt SJ: Microvessel density and distribution in ductal carcinoma in situ of the breast. J Natl Cancer Inst 86:614–619, 1994.
Arteaga CL, Carty-Dugger T, Moses HL, Hurd SD, Pietempol JA: Transforming growth factor-beta(1) can induce estrogen-independent tumorigenicity of human breast-cancer cells in athymic mice. Cell Growth Differ 4:193–201, 1993.
Costa M, Danesi R, Agen C, Di Paolo A, Basolo F, Del Bianchi S, Del Tacca M: MCF-10A cells infected with theint-2 oncogene induce angiogenesis in the chick chorioallantoic membrane and in the rat mesentery. Cancer Res 54:9–11, 1994.
Kurebayashi J, McLeskey SW, Johnson MD, Lippman ME, Dickson RB, Kern FG: Quantitative demonstration of spontaneous metastasis by MCF-7 human breast cancer cells cotransfected with fibroblast growth factor-IV and lacZ. Cancer Res 53:2178–2187, 1993.
McLeskey SW, Kurebayashi J, Honig SF, Zwiebel J, Lippman ME, Dickson RB, Kern FG: Fibroblast growth factor-IV transfection of MCF-7 cells produces cell lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude mice. Cancer Res 53:2168–2177, 1993.
Zajchowski DA, Band V, Trask DK, Kling D, Connolly JL, Sager R: Suppression of tumor-forming ability and related traits in MCF-7 human breast cancer cells by fusion with immortal mammary epithelial cells. Proc Natl Acad Sci USA 87:2314–2318, 1990.
Gasparini G, Pozza F, Harris AL: Evaluating the potential usefulness of new prognostic and predictive indicators in node-negative breast cancer patients [review]. J Natl Cancer Inst 85:1206–1219, 1993.
Grondahl-Hansen J, Christensen IJ, Rosenquist C, Brunner N, Mouridsen HT, Dano K, Blichert-Toft M: High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosol extracts of breast carcinomas are associated with poor prognosis. Cancer Res 53:2513–2521, 1993.
Janicke F, Schmitt M, Graeff H: Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Sem Thromb Hemostasis 17:303–332, 1991.
Foekens JA, Schmitt M, vanPutten WLJ, Peters HA, Kramer MD, Janicke F, Klijn JGM: Plasminogen activator inhibitor-1 and prognosis in primary breast cancer. J Clin Oncol 12:1648–1658, 1994.
Li V, Yu C, Rupnick M, Allred E, Sallan S, Hayes DF, Folkman J: Serum from breast cancer patients contains proliferative activity for capillary endothelial cells which correlates with risk of mortality [abstract #252]. Proc Am Soc Clin Oncol 12:113, 1993.
Nguyen M, Watanabe, Budson AE, Richie JP, Hayes DF, Folkman J: Elevated levels of an angiogenic peptide, basic fibroblast growth factor, in the urine of patients with a wide spectrum of cancers. J Natl Cancer Inst 86:356–361, 1994.
Horak ER, Leek R, Klenk N, Le Jeune S, Smith K, Stuard N, Greenall M, Stepniewska K, Harris AL: Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet 340:1120–1124, 1992.
Fox SB, Gatter KC, Bicknell R, Going JJ, Stanton P, Cooke TG, Harris AL: Relationship of endothelial cell proliferation to tumor vascularity in human breast cancer. Cancer Res 53:4161–4163, 1993.
Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G: Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 84:1875–1887, 1992.
Bevilacqua P, Boracchi P, Gasparini G: Prognostic indicators for early-stage breast carcinoma. Part II: Value of cathepsin D expression, detected by immunocytochemistry. A multiparametric study. Int J Oncol 5:559–564, 1994.
Weidner N, Gasparini G: Determination of epidermal growth factor receptor provides additional prognostic information to measuring tumor angiogenesis in breast carcinoma patients. Breast Cancer Res Treat 29:97–107, 1994.
Marx J: Cellular changes on the route to metastasis. Science 259:626–629, 1993.
Gasparini G, Barbareschi M, Boracchi P, Bevilacqua P, Verderio P, Dalla Palma P, Menard S: 67 kDa laminin receptor expression adds prognostic information to intra-tumoral microvessel density in node-negative breast cancer. Int J Cancer 60:604–610, 1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bevilacqua, P., Barbareschi, M., Verderio, P. et al. Prognostic value of intratumoral microvessel density, a measure of tumor angiogenesis, in node-negative breast carcinoma — results of a multiparametric study. Breast Cancer Res Tr 36, 205–217 (1995). https://doi.org/10.1007/BF00666041
Issue Date:
DOI: https://doi.org/10.1007/BF00666041