Abstract
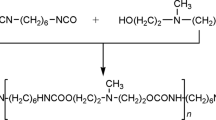
Natural montmorillonite, fractionated from bentonite produced in Yamagata, Japan, was ion-exchanged for NH +3 −(CH2)11−COOH, NH +3 −(CH2)5−COOH, Al3+, Cu2+, Mg2+, Co2+, Li+, K+ and H+. The mixtures of the ion-exchanged montmorillonite and ε-caprolactam were heated at 263°C in glass ampoules for various periods. The intercalated compounds before and after the heating were examined by X-ray powder diffraction, DSC and GPC. Although ε-caprolactam was not polymerized without montmorillonite, it was polymerized at 263°C in the presence of montmorillonite. The polymerization rate varied with the interlayer cations in the order of NH +3 −(CH2)11−COOH>Al3+>NH +3 −(CH2)5−COOH>H+>Cu2+>Mg2+>Co2+>Li+>K+. After heating at 263°C for 5 h, the mean number-average molecular weight was about 1.5×104. Although the interlayer distance of NH +3 −(CH2)11−COOH type montmorillonite/ε-caprolactam compound increased from 2.85 nm to 4.90 nm by heating at temperatures above the melting point of ε-caprolactam, those of other compounds were not changed. After heating at 263°C, an intercalated compound of montmorillonite and 6-polyamide, whose interlayer distance was more than 10 nm, was obtained. It is concluded that montmorillonite acts as a Brönsted acid and initiates the open ring polymerization of ε-caprolactam and that the driving force of swelling is the polymerization energy.
Similar content being viewed by others
References
P. J. Dodson and P. Somasundaran:J. Colloid Interface. Sci. 97 (1984), 481–487.
J. T. A. M. Weltzen, H. N. Stein, J. M. Stevels, and C. A. M. Siskens:J. Colloid Interface. Sci. 81 (1981), 455–467.
A. Yamagishi:J. Chromatogr. 262 (1983), 41–60.
Y. Fukushima, K. Mori, and A. Murase:J. Incl. Phenom. 2 (1984), 305–315.
N. Adachi, M. Koizumi, and F. Kanamaru:Kogyozairyo (Industrial Materials, in Japanese),25 (1977), 44.
G. Lagaly and K. Beneke:Am. Mineralogist 60 (1975), 650–658.
D. M. C. MacEwan and M. J. Wilson.Crystal Structures of Clay Minerals and their X-ray Indentification (Ed. G. W. Brindley and G. Brown) pp. 197–248, Mineralogical Society, London (1980).
J. M. Thomas:Intercalated Chemistry (Ed. M. S. Whittingham and A. J. Jacobson) pp. 55–100, Academic Press (1982).
K. Norrish:Disc. Faraday Soc. 18 (1954), 120–134.
C. R. Hubbard:Adv. X-Ray Anal. 26 (1983), 45.
Y. Fukushima, S. Inagaki and T. Okamoto:Nendo Kagaku (J. Clay Sci. Japan, in Japanese),26 (1986), 187.
Y. Fukushima, to be published in the Proc. of 5th International Symposium on Surfactants in Solution, Bordeaux, 1984.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fukushima, Y., Inagaki, S. Synthesis of an intercalated compound of montmorillonite and 6-polyamide. Journal of Inclusion Phenomena 5, 473–482 (1987). https://doi.org/10.1007/BF00664105
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00664105