Abstract
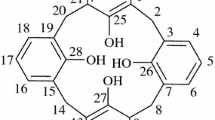
The configurations of calix[4]arenes may be modified by the formation of donor-acceptor complexes which make use of the basicity of the oxygen atoms of the macrocycle. The complex [t-butylcalix[4]arene methyl ether][AlMe3]2,2, exhibits the previously unseen ‘1,2-alternate’ geometry, while [t-butylcalix[4]arene methyl ether][MeAlCl2]2,3, and [t-butylcalix[4]arene methyl ether][EtAlCl2]2,4, show the ‘1,3-alternate’ configuration.2 crystallizes in the triclinic space groupPl witha=11.14(1),b=11.60(1),c=12.02(1) Å, α=77.32(8), β=67.91(8), and γ=69.34(8)o withD c =1.06 g cm−3 forZ=1. Refinement based on 1270 observed reflections led toR=0.106.3 as the benzene solvate belongs to the monoclinic space groupC2/c witha=12.116(2),b=21.557(7),c=23.470(6) Å, and β=104.05(2)o withD c =1.13 g cm−3 forZ=4. Refinement based on 2335 observed reflections led toR=0.075.4 crystallizes in the monoclinic space groupC2/c witha=12.062(4),b=21.175(6),c=21.596(5) Å, and β=100.78(4)o withD c =1.18 g cm−3 forZ=4. Refinement based on 2529 observed reflections gaveR=0.082. The Al-O lengths in all three complexes are normal for donor-acceptor interactions.
Similar content being viewed by others
References
C. D. Gutsche:Top. Curr. Chem. 123, 1 (1984).
V. Bocchi, D. Foina, A. Pochini, R. Ungaro and G. D. Andreetti:Tetrahedron 38, 373 (1982).
J. W. Cornforth, P. D'Arcy Hart, G. A. Nicholls, R. J. W. Rees and J. A. Stock:Brit. J. Pharmacol. 10, 73 (1955).
C. D. Gutsche, B. Dhawan, J. A. Levine, K. H. No and L. J. Bauer:Tetrahedron 39, 409 (1983).
A. W. Coleman, S. G. Bott and J. L. Atwood:J. Incl. Phenom. 5, 581 (1987).
The synthetic procedure was an adaptation of that for calix[6]arene. C. D. Gutsche and P. F. Pagoria:J. Org. Chem. 50, 5799 (1985).
The MeAlCl2 unit was generated from methylaluminum sesquichloride in the normal fashion. See, for example, T. Mole and E. A. Jeffrey:Organoaluminum Compounds, Elsevier, Amsterdam, 1972.
J. Holton, M. F. Lappert, D. G. H. Ballard, R. Pearce, J. L. Atwood, and W. E. Hunter:J. Chem. Soc., Dalton Trans. 45 (1979).
G. Germain, P. Main, and M. M. Woolfson:Acta Crystallogr. A 27, 368 (1971).
SHELX, a system of computer programs for X-ray structure determination by G. M. Sheldrick (1976).
D. T. Cromer and J. T. Waber:acta Crystallogr. 18, 104 (1965).
J. A. Ibers and W. C. Hamilton (eds).International Tables for X-ray Crystallography vol. IV, p. 72, Kynoch Press, Birmingham, England, 1974 Distr. D. Reidel Publ. Co., Dordrecht, The Netherlands.
G. H. Robinson, S. G. Bott, H. Elgemal, W. E. Hunter, and J. L. Atwood:J. Incl. Phenom. 3, 65 (1985).
A. Vrielink, P. W. Codding, C. D. Gutsche, and L.-G. Lin:J. Incl. Phenom. 4, 199 (1986).
S. G. Bott, H. Elgemal, and J. L. Atwood:J. Am. Chem. Soc. 107, 1796 (1985).
A. W. Coleman and J. L. Atwood: Unpublished results.
J. L. Atwood inInclusion Compounds, vol. 1, J. L. Atwood, D. D. MacNicol and J. E. D. Davies (eds.), Academic Press, London, 1984.
J. L. Atwood, D. C. Hrncir, R. Shakir, M. S. Dalton, R. D. Priester, and R. D. Rogers:Organometallics 1, 1021 (1982).
A. Bondi:J. Chem. Phys. 68, 441 (1964).
S. R. Izatt, R. J. Hawkins, J. J. Christensen, and R. M. Izatt:J. Am. Chem. Soc. 107, 63 (1985).
S. G. Bott, A. W. Coleman, and J. L. Atwood:J. Am. Chem. Soc. 108, 1709 (1986).
S. G. Bott and J. L. Atwood: Unpublished results and C. Rizzoli, G. D. Andreetti, R. Ungaro, and A. Pochini.J. Mol. Struct. 82, 133 (1982).
Author information
Authors and Affiliations
Additional information
Supplementary Data relating to this article are deposited with the British Library as Supplementary Publication No. SUP 82053 (45 pages).
Rights and permissions
About this article
Cite this article
Bott, S.G., Coleman, A.W. & Atwood, J.L. Alternative methods of modifying the calixarene conformation. The synthesis and molecular structures oft-butylcalix[4]arene methyl ether complexed with aluminum alkyl species. Journal of Inclusion Phenomena 5, 747–758 (1987). https://doi.org/10.1007/BF00656595
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00656595