Abstract
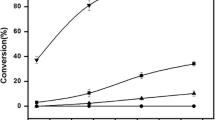
Chiral esters with high optical purity have been synthesized at 298.2 K from racemic 2-octanol and alkanoic acids using the commerical lipases fromChromobacterium viscosum (CV) orCandida sp. (SP 525) immobilized in microemulsion-based gelatin gels. The microemulsions consisted of water and alkanes stabilized by the anionic surfactant sodium 1,4-bis(2-ethylhexyl) sulfosuccinate (AOT) and the naturally occurring zwitterionic surfactant soybean lecithin, respectively. The enzymes were solubilized both in water-in-oil (W/O) microemulsions and in microemulsions with a bicontinuous structure. Different microstructures of the gels were chosen since the enzyme may undergo conformational changes in different environments resulting in different catalytic efficiencies toward competing substrates. Therefore, it is of great fundamental interest to know the phase behaviour and the microstructures of the used microemulsion systems. Phase diagrams were determined at 298.2 K for the systems water-hexane-AOT and ethanol/water (1∶1)-hexadecane-soybean lecithin. The former system exhibited a large one-phase W/O microemulsion region, while in the latter a small one-phase region with bicontinuous structure was present. The kinetic enantiomeric ratios (E-values), as determined from enantiomeric excess (e.e.) values at a conversion below 0.5, were higher both in the W/O microemulsion as well as in the bicontinuous microemulsion using the SP 525 lipase, than using the CV lipase. On the other hand, the conversions were higher using gels based on W/O microemulsions (AOT stabilized) than using gels based on microemulsions with a bicontinuous structure (lecithin stabilized).
Similar content being viewed by others
References
Danielsson I, Lindman B (1981) Colloids Surfaces 3:391–392
Stilbs P, Lindman B (1984) Progr Colloid Polym Sci 69:39–47
Evans DF, Mitchell DJ, Ninham BW (1986) J Phys Chem 90:2817–2825
Israelachvili J (1994) Colloids Surfaces A: Physicochemical Eng Aspects 91:1–8
Zana R (1994) Heterogeneous Chem Rev 1:145–157
Stickdorn K, Schwuger MJ, Schomäcker R (1994) Tenside Surf Det 31:218–228
Fletcher PDI, Freedman RB, Mead J, Oldfield C, Robinson BH (1984) Colloids Surfaces 10:193–203
Fletcher PDI, Robinson BH, Freedman RB, Oldfield C (1985) J Chem Soc Faraday Trans 181:2667–2679
Fletcher PDI, Freedman RB, Robinson BH, Rees GD, Schomäcker R (1987) Biochim Biophys Acta 912:278–282
Schomäcker R, Robinson BH, Fletcher PDI (1988) J Chem Soc Faraday Trans 184:4203–4212
Xenakis A, Valis TP, Kolisis FN (1989) Progr Colloid Polym Sci 79:88–93
Larsson KM, Oldfield C, Freedman RB (1989) Eur J Biochem 183:357–361
Larsson KM, Olsson U, Adlercreutz P, Mattiasson B (1990) Biotechnol Bioeng 35:135–141
Stark MB, Skagerlind P, Holmberg K, Carlfors J (1990) Colloid Polym Sci 268:384–388
Hayes DG, Gulari E (1990) Biotechnol Bioeng 35:793–801
Larsson KM, Adlercreutz P, Mattiasson B (1991) J Chem Soc Faraday Trans 87:465–471
Kolisis FN, Valis TP, Xenakis A (1990) Ann NY Acad Sci 613:674–680
Xenakis A, Valis TP, Kolisis N (1991) Progr Colloid Polym Sci 84:508–511
Hedström G, Slotte JP, Backlund M, Molander O, Rosenholm JB (1992) Biocatalysis 6:281–290
Hedström G, Slotte JP, Molander O, Rosenholm JB (1992) Biotechnol Bioeng 39:218–224
Hayes DG, Gulari E (1992) Biotechnol Bioeng 40:110–118
Skagerlind P, Jansson M, Hult K (1992) J Chem Tech Biotechnol 54:277–282
Miyake Y, Owari T, Matsuura K, Teramoto M (1993) J Chem Soc Faraday Trans 89:1993–1999
Pileni MP (1993) J Phys Chem 97:6961–6973
Pileni MP (1993) Adv Colloid Interface Sci 46:139–163
Hedström G, Backlund M, Slotte JP (1993) Biotechnol Bioeng 42:618–624
Singh CP, Shah DO (1993) Colloids Surfaces A: Physicochem Eng Aspects 77:219–224
Yang CL (1993) Ph D Thesis, The University of Michigan, USA
Xenakis A, Stamatis H, Malliaris A, Kolisis FN (1993) Progr Colloid Polym Sci 93:373–376
Miyake Y, Owari T, Ishiga F, Teramoto M (1994) J Chem Soc Faraday Trans 90:975–986
Singh CP, Skagerlind P, Holmberg K, Shah DO (1994) J Amer Oil Chem Soc 71:1405–1409
Skagerlind P, Holmberg K (1994) J Dispersion Sci Technol 15:317–332
Backlund S, Rantala M (1995) Colloid Polym Sci 273:293–297
Backlund S, Eriksson F, Karlsson S, Lundsten G (1995) Colloid Polym Sci 273:533–538
Sonesson C, Holmberg K (1991) J Colloid Interface Sci 141:239–244
Holmberg K (1994) Adv Colloid Interface Sci S1:137–174
Dodson GG, Lawson DM, Winkler FK (1992) Faraday Discuss 93:95–105
Rees GD (1990) Ph D Thesis, University of East Anglia
Rees GD, Nascimento MG, Jenta TRJ, Robinson BH (1991) Biochim Biophys Acta 1073:493–501
Rees GD, Robinson BH (1993) Adv Mater 5:608–619
Rees GD, Jenta TRJ, Nascimento MG, Catauro M, Robinson BH, Stephenson GR, Olphert RDG (1993) Indian J Chem 32B:30–34
Jenta TRJ, Robinson BH, Batts G, Thomson AR (1991) Progr Colloid Polym Sci 84:334–337
Nascimento MG, Rezende MC, Vecchia RD, Jesus PC, Aguiar LMZ (1992) Tetrahedron Lett 33:5891–5894
Uemasu I, Hinze WL (1994) Chirality 6:649–653
Jesus PC, Rezende MC, Nascimento MG (1995) Tetrahedron Asymmetry 6:63–66
Backlund S, Eriksson F, Kanerva LT, Rantala M (1995) Colloids Surfaces B: Biointerfaces 4:121–127
La Mesa C, Coppola L, Rainieri GA, Terenzi M, Chidichimo G (1992) Langmuir 8:2616–2622
Shinoda K, Araki M, Sadaghiani A, Khan A, Lindman B (1991) J Phys Chem 95:989–993
Shinoda K, Shibata Y, Lindman B (1993) Langmuir 9:1254–1257
Backlund S, Rantala M, Molander O (1994) Colloid Polym Sci 272:1098–1103
Ohshima A, Norita H, Kito M (1982) J Biochem 93:1421–1425
Morita S, Norita H, Matoba T, Kito M (1984) J Amer Oil Chem Soc 61:1571–1574
Schmidli PK, Luisi PL (1990) Biocatalysis 3:367–376
Haering G, Luisi PL (1986) J Phys Chem 90:5892–5895
Quellet C, Eicke HP (1986) Chimia 40:233–238
Robinson BH (1990) Chem Br 342–344
Chen CS, Fujimoto Y, Girdaukas G, Sih CJ (1982) J Amer Chem Soc 104:7294–7299
Jada A, Lang J, Zana R (1989) J Phys Chem 93:10–12
Kunieda H, Nakamura K, Davis HT, Evans DF (1991) Langmuir 7:1915–1991
Backlund S, Eriksson F, Kanerva LT, Karlsson S, Lundsten G, Rantala M, Vänttinen E, Wahtera G (1994) In: Stenius P, Sarvaranta L (eds) 12th Scand Symp Surface Chem, TKK Offset, Espoo 1994, Series C6:117–119
Petit C, Zemb T, Pileni MP (1991) Langmuir 7:223–231
Chen CS, Wu SH, Girdaukas G, Sih CJ (1987) J Amer Chem Soc 109:2812–2817
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Backlund, S., Eriksson, F., Hedström, G. et al. Lipase-catalyzed enantioselective esterifications using different microemulsion-based gels. Colloid Polym Sci 274, 540–547 (1996). https://doi.org/10.1007/BF00655229
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00655229