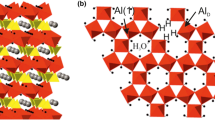
Abstract
High-resolution aluminum-27 and silicon-29 nuclear magnetic resonance spectra of natural and synthetic imogolites and allophanes obtained using high-field“magic-angle” sample-spinning (MASS) techniques indicate that the imogolite and protoimogolite components of allophanes are characterized by sharp (≈3 ppm) silicon-29 resonances at −78±1 ppm from tetramethylsilane (in accord with Barron et al. 1982), and quite narrow (≈10 ppm at 11.7 Tesla) aluminum-27 resonances, at 5.2±1 ppm from Al(H2O) 3+6 (in accord with Wilson et al. 1984). However, the spectra of natural allophanes usually contain significant intensity arising from a less well defined material, characterized by a broad (≈20 ppm) silicon-29 resonance centered at −90±2 ppm from tetramethylsilane, and a second relatively narrow (≈15 ppm at 11.7 Tesla) aluminum-27 resonance at 58.5±2 ppm from Al(H2O) 3+6 . Similar characteristic spectral features are exhibited by a synthetic amorphous Si:Al (1:1) gel, and presumably indicate the presence of framework aluminosilicate materials in the gel, and in most allophanes.
Similar content being viewed by others
References
Alma NCM, Hays GR, Samoson AV, Lippmaa ET (1984) Characterization of synthetic dioctahedral clays by solid state silicon-29 and aluminum-27 nuclear magnetic resonance spectrometry. Anal Chem 56:729–733
Andrew ER (1971) The narrowing of NMR spectra of solids by high-speed specimen rotation and resolution of chemical shift and spin multiplet structures for solids. Progr NMR Spectrosc 8:1–39
Barron PF, Wilson MA, Campbell AS, Frost RL (1982) Detection of imogolite in soils using solid state29Si NMR. Nature 299:616–618
Cradwick PDG, Farmer VC, Russell JD, Masson CR, Wada K, Yoshinaga N (1972) Imogolite, a hydrated aluminium silicate of tubular structure. Nature Phys Sci 240:187–189
de Jong BHWS, Schramm CM, Parziale VE (1983) Polymerization of silicate and aluminate tetrahedra in glasses, melts and aqueous solutions. V. Aluminum coordination in glasses and aqueous solutions and comments on the aluminum avoidance principle. Geochim Cosmochim Acta 47:1223–1236
Farmer VC (1981) Possible roles of mobile hydroxyaluminium orthosilicate complex (proto-imogolite) in podzolization. In: Migration organo-minerales dans les sols temperes. International Colloquium of CNRS Nancy, No 303:275–279
Farmer VC (1982) Significance of the presence of allophane and imogolite in podzol Bs horizons for podzolization mechanisms: a review. Soil Sci Plant Nutr 28:571–578
Farmer VC, Fraser AR (1979a) Synthetic imogolite, a tubular hydroxyaluminium silicate. In: Mortland MM, Farmer VC (eds) International Clay Conference, Oxford, 1978, Elsevier, Amsterdam, pp 547–553
Farmer VC, Fraser AR, Tait JM (1979b) Characterization of the chemical structures of natural and synthetic aluminosilicate gels and sols by infrared spectroscopy. Geochim Cosmochim Acta 43:1417–1420
Farmer VC, Fraser AR, Russell JD, Yoshinaga N (1977) Recognition of imogolite structures in allophanic clays by infrared spectroscopy. Clay Miner 12:55–57
Farmer VC, Russell JD, Berrow ML (1980) Imogolite and proto-imogolite allophane in spodic horizons: evidence for a mobile aluminium silicate complex in podzol formation. J Soil Sci 31:673–684
Grimmer A-R, von Lampe F, Mägi M, Lippmaa E (1983) Solid state high resolution silicon-29 NMR of silicates; effect of iron (2+) in olivines, Z Chem 23:343–344
Janes N, Oldfield E (1985) Prediction of silicon-29 nuclear magnetic resonance chemical shifts using a group electronegativity approach: applications to silicate and aluminosilicate structures. J Am Chem Soc, in press
Kinsey RA, Ph.D. Thesis, University of Illinois, 1984
Kinsey RA, Kirkpatrick RJ, Hower J, Smith KA, Oldfield E (1985) High resolution aluminium-27 and silicon-29 nuclear magnetic resonance spectroscopic study of layer silicates, including clay minerals. Am Mineral 70:537–548
Klinowski J, Thomas JM, Fyfe CA, Hartman JS (1981) Applications of magic-angle-spinning silicon-29 nuclear magnetic resonance. Evidence for two different kinds of silicon-aluminium ordering in zeolitic structures. J Phys Chem 85:2590–2594
Lippmaa E, Mägi M, Samoson A, Engelhardt G, Grimmer A-R (1980) Structural studies of silicates by solid-state high-resolution29Si NMR. J Am Chem Soc 102:4889–4893
Lippmaa E, Mägi M, Samoson A, Tarmak M, Engelhardt G (1981) Investigation of the structure of zeolites by solid-state high resolution29Si NMR spectroscopy. J Am Chem Soc 103:4992–4996
Maciel GE, Sindorf DW (1980) Silicon-29 nuclear magnetic resonance study of the surface of silica gel by cross polarization and magic-angle spinning. J Am Chem Soc 102:7606–7607
MacKenzie KJD (1970) Thermal decomposition of Derbyshire allophane. Clay Miner 8:349–351
Mitchell BD, Farmer VC, McHardy WJ (1964) Amorphous inorganic materials in soil. Adv Agron 16:327–375
Müller D, Gessner W, Behrens H-J, Scheler G (1981) Determination of the aluminium coordination in aluminium-oxygen compounds by solid-state high-resolution27Al NMR. Chem Phys Letters 79:59–62
Oldfield E, Kinsey RA, Smith KA, Nichols JA, Kirkpatrick RJ (1983) High resolution NMR of inorganic solids. Influence of magnetic centers on magic angle sample-spinning lineshapes in some natural aluminosilicates. J Magn Reson 51:325–329
Parfitt RL, Furkert RJ, Henmi T (1980) Identification and structure of two types of allophane from volcanic ash soils and tephra. Clays Clay Miner 28:328–334
Ross CS, Kerr PF (1934) Halloysite and allophane, US Geol Surv Prof Paper 185G:135–148
Russell JD, McHardy WJ, Fraser AR (1969) Imogolite: a unique aluminosilicate. Clay Miner 8:87–99
Smith KA, Kirkpatrick RJ, Oldfield E, Henderson DM (1983) High resolution silicon-29 nuclear magnetic resonance spectroscopic study of rock-forming silicates. Am Mineral 68:1206–1215
Wells N, Childs CW, Downes CJ (1977) Silica Springs, Tongariro National Park, New Zealand — analyses of the spring water and characterization of the alumino-silicate deposit. Geochim Cosmochim Acta 41:1497–1506
Wilson MA, Barron PF, Campbell AS (1984) Detection of aluminum coordination in soils and clay fractions using27Al magic angle spinning n.m.r. J Soil Sci 35:201–207
Yoshinaga N, Aomine S (1962) Allophane in some Ando soils. Soil Sci Plant Nutr 8:6–13
Yoshinaga N, Yamaguchi M (1970) Occurrence of imogolite as gel film in the pumice and scoria beds of Western and Central Honshu and Hokkaido. Soil Sci Plant Nutr 16:215–223
Young AW, Campbell AS, Walker TW (1980) Allophane isolated from a podsol developed on a non-vitric parent material. Nature 284:46–48
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goodman, B.A., Russell, J.D., Montez, B. et al. Structural studies of imogolite and allophanes by aluminum-27 and silicon-29 nuclear magnetic resonance spectroscopy. Phys Chem Minerals 12, 342–346 (1985). https://doi.org/10.1007/BF00654344
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00654344