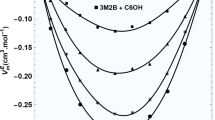
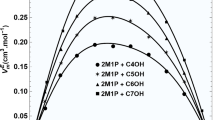
Abstract
A recently developed model for 1-alkanol+alkane mixtures is extended to methanol mixtures and to the non-polar mixing partners tetrachloromethane and benzene. The model contains chemical and physical terms, which are combined in a thermodynamically consistent way. For our calculations on methanol mixtures, we have measured gE of methanol+ hexane via static vapor pressure measurements. In order to check the model predictions for systems with higher alkanols and alkanes, we have also determined gE of 1-octanol+tetradecane by measuring the melting curve. The reproduction of the excess properties of methanol+hexane, the agreement between predicted and measured values of gE for 1-octanol+ tetradecane, and the capability to deal also with other non-polar mixing partners demonstrate the power and reliability of the model.
Similar content being viewed by others
References
A. Liu, F. Kohler, L. Karrer, J. Gaube, and P. Spellucci,Pure and Appl. Chem. 61, 1441 (1989).
J. A. Riddick, W. B. Bunger, and T. K. Sakaro,Organic Solvents, 4th edn., (Wiley, New York, 1986).
T. Boublik and K. Aim,Coll. Czech. Chem. Comm. 37, 3513 (1972).
T. M. Letcher and F. Marsicano,J. Chem. Thermodyn. 6, 509 (1974).
R. R. Dreisbach and R. A. Martin,Ind. Eng. Chem. 41, 2875 (1949).
A. J. Streiff, A. R. Hulme, P. A. Cowie, N. C. Krouskop, and F. D. Rossini,Anal. Chem. 27, 411 (1955).
H. Atrops, H. E. Kalali, and F. Kohler,Ber. Bunsenges. Phys. Chem. 86, 26 (1982).
M. Davies and B. Kybett,Trans. Faraday Soc. 61, 1608 (1965).
J. K. Cline and D. H. Andrews,J. Am. Chem. Soc. 53, 3668 (1931).
H. L. Finke, M. E. Gross, G. Waddington, and H. M. Huffman,J. Am. Chem. Soc. 76, 333 (1954).
J. F. Messerly and G. B. Guthrie,J. Chem. Eng. Data 12, 338 (1967).
G. S. Parks and D. W. Light,J. Am. Chem. Soc. 56, 1511 (1934).
N. Van Nhu, G. Nowak, and P. Svejda,Ber. Bunsenges. Phys. Chem. 92, 1537 (1988).
A. Liu, Doctoral Thesis, Ruhr-University, Bochum (1990).
N. Van Nhu and F. Kohler,Fluid Phase Equili. 50, 267 (1989).
M. A. Siddiqi, G. Götze, and F. Kohler,Ber. Bunsenges. Phys. Chem. 84, 529 (1980).
H. E. Kalali, A. M. Demiriz, J. Budde, F. Kohler, A. Dallos, and F. Ratkovics,Fluid Phase Equili. 54, 111 (1990).
K. N. Marsh and A. E. Richards,J. Chem. Eng. Data 23, 288 (1978).
B. D. Smith and R. Srivastava,Thermodynamic Data for Pure Compounds, Part B (Elsevier, Amsterdam 1986).
J. H. Dymond and E. B. Smith,The Virial Coefficients of Pure Gases and Mixtures (Clarendon Press, Oxford 1980).
J. J. Ott, G. V. Cornett, C. E. Stouffer, B. F. Woodfield, C. Guanquan, and J. J. Christensen,J. Chem. Thermodyn 18, 867 (1986).
S. C. Hwang and R. L. Robinson,J. Chem. Eng. Data 22, 319 (1977).
H. C. Van Ness and M. M. Abbott,Int. Data Ser. A 2 (1976).
G. Hradetzky and H.-J. Bittrich,Int. Data Ser. A 216 (1986).
F. Kohler,Monatsh. Chem. 88, 388 (1957).
R. J. Munn and F. Kohler,Monatsh. Chem. 91, 381 (1960).
V. Ragaini, R. Santi, and S. Carrà,Lincei Rend. Sc., Fis. Mat. e Nat. 45, 540 (1968).
E. S. Kim and K. N. Marsh,J. Chem. Eng. Data 33, 288 (1983).
M. D. Donohue and J. M. Prausnitz,Can. J. Chem. 53, 1586 (1975).
H. V. Kehiaian,Ber. Bunsenges. Phys. Chem. 81, 908 (1977).
H. V. Kehiaian, J.-P. E. Grolier, and G. C. Benson,J. Chim. Physique 75, 1031 (1978).
L. Andreoli-Ball, D. Patterson, M. Costas, and M. Caceres-Alonso,J. Chem. Soc. Faraday Trans. I. 84, 3991 (1988).
J. A. Barker, I. Brown, and F. Smith,Disc. Faraday Soc. 15, 142 (1953).
R. M. Stokes and C. Burfitt,J. Chem. Thermodyn. 5, 623 (1973).
Y. S. Luo, P. L. Cen, B. G. Li, J. D-Zhou, and Z. Q. Zhu,Fluid Phase Equili. 20, 137 (1985).
T. B. Tai, R. S. Ramalho, and S. Kaliaguine,Can. J. Chem. Eng. 50, 771 (1972).
G. Scatchard and L. Ticknor,J. Am. Chem. Soc. 74 3724 (1952).
E. A. Moelwyn-Hughes and R. W. Missen,J. Phys. Chem. 61, 518 (1957).
G. C. Paraskevopoulos and R. W. Missen,Trans. Faraday Soc. 58, 869 (1962).
R. F. Platford,J. Chem. Soc. Faraday Trans. I 73, 267 (1977).
B. Darce and G. C. Benson,Can. J. Chem. 41, 278 (1963).
I. Nagata and K. Tamura,Fluid Phase Equili. 15, 67 (1963).
J.-E. Otterstedt and R. W. Missen,Trans. Faraday Soc. 58, 879 (1962).
G. Scatchard, S. E. Wood, and J. M. Mochel,J. Am. Chem. Soc. 8, 1957 (1946).
K. Strubl, V. Svoboda, and R. Holub,Coll. Czech. Chem. Comm. 37, 3522 (1972).
A. Oracz and G. Kolassinska,Fluid Phase Equili. 35, 253 (1987).
V. C. Smith and R. L. Robinson,J. Chem. Eng. Data 15 391 (1970).
R. F. Platford,J. Solution Chem. 5, 645 (1976).
K. Hoskyns, D. Jones, and B. C.-Y. Lu,J. Chem. Eng. Data 11, 488 (1966).
H. W. Schnaible, H. C. Van Ness, and J. M. Smith,AIChE J. 3, 147 (1957).
F. Vesely, V. Hynek, V. Svoboda, and R. Holub,Coll. Czech. Chem. Comm. 39, 355 (1974).
C. C. Tsao and J. M. Smith,Chem. Eng. Progr. Symp. Ser. 49, 197 (1953).
G. C. Williams, S. Rosenberg, and H. A. Rothenberg,Ind. Eng. Chem. 40, 1273 (1948).
P. S. Murti and M. Van Winkle,J. Chem. Eng. Data 3 65 (1958).
D. Bares, M. Soulie, and J. Metzger,J. Chim. Physique Biol. 70, 1531 (1973).
I. Brown and W. Fock,Austral. J. Chem. 14, 387 (1961).
J. R. Goates, R. L. Snow, and M. R. James,J. Phys. Chem. 65 335 (1961).
E. R. Washburn and A. Lightbody,J. Phys. Chem. 34, 2701 (1930).
R. V. Mrazek and H. C. Van Ness,AIChE J. 7, 190 (1961).
F. Vesely and J. Pick,Coll. Czech. Chem. Comm. 34, 1854 (1969).
K.-Y. Hsu and H. L. Clever,J. Chem. Eng. Data 20, 268 (1975).
Author information
Authors and Affiliations
Additional information
Communicated at the Festsymposium celebrating Dr. Henry V. Kehiaian's 60th birthday, Clermont-Ferrand, France, 17–18 May 1990.
Rights and permissions
About this article
Cite this article
Liu, A., Pusicha, K., Demiriz, A.M. et al. Model for alkanol + alkane mixtures: Extension and experimental verification. J Solution Chem 20, 39–56 (1991). https://doi.org/10.1007/BF00651639
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00651639