Abstract
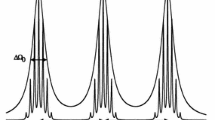
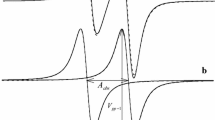
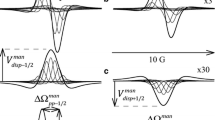
The ESR spectrum of the nitroxide radical 2,2,6,6-tetramethylpiperidine-N-oxide (TEMPO) has been investigated in aqueous solutions of the following surfactants:n-octylammonium bromide, sodiumn-octylsulfonate,n-decylammonium bromide,n-docecyltrimethylammonium bromide, and sodiumn-dodecylsulfate. The spectra were recorded at 25° C as function of surfactant concentration in the ranges 0–0.5m for the C10 and C12 compounds and 0–1.0m for the others. Analysis of the spectra using computer simulation methods yields the hyperfine coupling constanta H and the widthW H of the partially resolved lines in the proton hyperfine structure. Changes in these variables with surfactant concentration are interpreted from medium effects on the magnetic properties of the radical, kinetics of radical-micelle interactions, and rotational dynamics of the nitroxide probe in these solutions.
Similar content being viewed by others
References
T. J. T. Buckman, P. L. Nordio, and H. M. McConnell,Proc. Nat. Acad. Sci. USA 54, 1010 (1965).
J. Oakes,J. Chem. Soc. Faraday Trans. 2 68, 1464 (1972);69, 1321 (1972).
O. H. Griffith and A. S. Waggoner,Acc. Chem. Res. 2, 17 (1969).
J. Martinie, J. Michon, and A. Rassat,J. Am. Chem. Soc. 97, 1818 (1975).
T. Nakagawa and H. Jizomoto,Kolloid Z. Z. Polym. 250, 594 (1972).
N. M. Atherton and S. J. Strach,J. Chem. Soc. Faraday Trans. 2 68, 1 (1972).
J. R. Ernandes, S. Schrier, and H. Chaimovich,Chem. Phys. Lipids 16, 19 (1976).
J. C. Lang and J. H. Freed,J. Chem. Phys. 56, 4103 (1972).
S. Ablett, M. D. Barratt, and F. Franks,J. Solution Chem. 4, 797 (1975).
K. K. Fox,Trans. Faraday Soc. 67, 2802 (1971);Chemical and Biological Applications of Relaxation Spectrometry, E. Wynn-Jones, ed. (D. Reidel Publishing Co., Dordrecht, 1975), p. 215.
C. Jolicoeur and H. L. Friedman,Ber. Bunsenges. Phys. Chem. 75, 248 (1971).
C. Jolicoeur and H. L. Friedman,J. Solution Chem. 3, 15 (1974).
B. L. Bales, J. A. Swenson, and R. N. Schwartz,Mol. Cryst. Liq. 28, 143 (1974).
P. Devaux, C. J. Scandella, and H. M. McConnell,J. Magn. Reson. 9, 474 (1973); E. Sackmann and H. Trauble,J. Am. Chem. Soc. 94, 4482 (1972).
I. C. P. Smith,Biological Applications of Electron Spin Resonance Spectroscopy, J. R. Bolton, D. Borg, and H. Schwartz, eds. (Wiley-Interscience, New York, 1971).
H. Dugas,Acc. Chem Res. 10, 47 (1977).
A. H. Cohen and B. M. Hoffman,J. Phys. Chem. 78, 1314 (1974).
G. Stout and J. B. F. N. Engberts,J. Org. Chem. 39, 3800 (1974).
C. C. Wishnant, S. Ferguson, and D. B. Chesnut,J. Phys. Chem. 78, 1410 (1974).
A. E. Stillman and R. N. Schwartz,J. Magn. Resan. 22, 269 (1976).
G. Poggi and C. S. Johnson, Jr.,J. Magn. Reson. 3, 436 (1970).
E. G. Rozantzev and M. B. Neiman,Tetrahedron 20, 131 (1964).
D. Kivelson,J. Chem. Phys. 33, 1094 (1960).
P. Mukerjee and K. J. Mysels,Critical Micelle Concentrations of Aqueous Surfactants Systems, National Bureau of Standards, Publication No. 36, U.S. Dept. of Commerce, Washington, D.C.;
K. Shinoda, T. Nakagawa, B. Tamamushi, and T. Isemura,Colloidal Surfactants (Academic Press, New York, 1963);
J. E. Desnoyers and M. Arel,Can. J. Chem. 43, 359 (1967);
W. Brunig and A. Holtzer,J. Am. Chem. Soc. 83, 4865 (1961).
E. Graber, J. Lang, and R. Zana,Kolloid Z. Z. Polym. 238, 470 (1970).
J. Rassing, P. Sams, and E. Wynn-Jones,J. Chem. Soc. Faraday Trans. 1 69, 180 (1973).
J. Lang, C. Tondre, R. Zana, R. Bauer, H. Hoffmann, and W. Ulbricht,J. Phys. Chem. 79, 276 (1975).
N. Muller,J. Phys. Chem. 76, 3017 (1972).
T. Nakagawa,Colloid Polym. Sci. 252, 56 (1974).
P. J. Sams, E. Wynn-Jones, and J. Rassing,Chem. Phys. Lett. 13, 233 (1972).
R. Folger, H. Hoffmann, and W. Ulbricht,Ber. Bursenges. Phys. Chem. 78, 986 (1974).
E. A. G. Aniansson and S. N. Wall,J. Phys. Chem. 78, 1024 (1974).
E. A. G. Aniansson, S. N. Wall, M. Almgren, H. Hoffmann, I. Kielman, W. Ulbricht, R. Zana, J. Lang, and C. Tondre,J. Phys. Chem. 80, 905 (1976).
K. M. Salikhov, A. B. Doctorov, and Yu. N. Molin,J. Magn. Reson. 5, 189 (1971).
J. Brotherus and P. Tormala,Kolloid Z. Z. Polym. 251, 774 (1973).
K. Shirahama, M. Hayashi, and R. Matuura,Bull. Chem. Soc. Jpn. 42, 1206 (1969);42, 2123 (1969).
A. Abragam,The Principles of Nuclear Magnetism (Oxford University Press, London, 1961), pp. 114, 115, 272.
L. Libertini and O. H. Griffith,J. Chem. Phys. 53, 1359 (1970).
S. Meiboom, Z. Luz, and D. Gill,J. Chem. Phys. 27, 1411 (1957).
T. A. Miller, R. N. Adams, and P. M. Richards,J. Chem. Phys. 44, 4022 (1966).
C. F. Polnaszek and J. H. Freed,J. Phys. Chem. 79, 2283 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jolicoeur, C., Friedman, H.L. ESR lineshapes and kinetic behavior of nitroxide spin probes in micellar solutions. J Solution Chem 7, 813–835 (1978). https://doi.org/10.1007/BF00650810
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00650810