Abstract
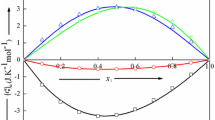
Phase diagrams, volumes and heat capacities of aqueous mixtures of 2,6-dimethylpyridine (2,6-L) and 2-isobutoxyethanol (iBE) and activities of 2,6-L in aqueous mixtures were measured in the monophasic region near the lower critical solution temperature (LCST). With 2,6-L some measurement were also made just above the LCST. From the temperature dependence of these data, partial molar relative enthalpies (2,6-L), expansibilities and the temperature derivative of heat capacities were calculated and show that iBE undergoes a microphase transition at low concentration which is not related to the phase separation. On the other hand, the properties of 2,6-L in the water-rich region at temperatures well below the LCST indicates that this solute has only a slight tendency to associate. The heat capacities of 2,6-L show an important increase near the LCST. Such changes are not observed for iBE and other alkoxyethanols and amines since these systems already exist in the form of microphases; the partial molar properties of iBE near the LCST are nearly equal to the molar values of the pure liquid, and the changes in thermodynamic properties corresponding to the macroscopic phase transition, are therefore too small to be measured by the present techniques.
Similar content being viewed by others
References
G. Roux, G. Perron, and J. E. Desnoyers,J. Solution Chem. 7, 639 (1978).
G. Roux, D. Roberts, G. Perron, and J. E. Desnoyers,J. Solution Chem. 9, 629 (1980).
M. Privat, L. Tenebre, R. Bennes, E. Tronel-Peyroz, J. M. Douillard, and L. Ghaicha,Langmuir 4, 1151 (1988).
D. Beysens and D. Estève,Phys. Rev. Lett. 54, 2123 (1985).
L. Ghaicha, M. Privat, L. Tenebre, R. Bennes, E. Tronel-Peyroz, and J. M. Douillard,Langmuir 4, 1326 (1988).
D. W. Pohl and W. I. Goldburg,Phys. Rev. Lett. 48, 1111 (1982).
U. Kaatze and D. Woermann,J. Phys. Chem. 88, 284 (1984).
U. Kaatze, Chr. Neumann, and R. Pottel,J. Solution Chem. 16, 191 (1987).
W. Mayer and D. Woermann,Ber. Bunsenges. Phys. Chem. 94, 145 (1990).
M. Jungk, L. Belkoura, and D. Woermann,Ber. Bunsenges Phys. Chem. 91, 507 (1987).
N. Ito and T. Kato,J. Phys. Chem. 88, 801 (1984).
E. Gulari, A. F. Collings, R. L. Schmidt, and C. J. Pings,J. Chem. Phys. 56, 6169 (1972).
A. Stein, S. J. Stevens, J. C. Allegra, and G. F. Allen,J. Chem. Phys. 56, 6164, (1972).
V. P. Gutschick and C. J. Pings,J. Chem. Phys. 55, 3845 (1971).
F. Quirion, L. J. Magid, and M. Drifford,Langmuir 6, 244 (1990).
G. D'Arrigo, F. Mallamace, N. Micali, A. Paparelli, J. Teixeira, and C. Vasi,Prog. Colloid Polym. Sci. 84, 177 (1991).
H. L. Cox and L. H. Cretcher,J. Am. Chem. Soc. 48, 451 (1926).
H.-J. Zimmer and D. Woermann,Ber. Bunsenges. Phys. Chem. 95, 533 (1991).
Y. Shindo and K. Kusano,J. Chem. Eng. Data. 24, 106 (1979).
R. J. Fanning and P. Kruus,Can. J. Chem. 48, 2052 (1972).
S. Nishikawa and T. Uchida,J. Solution Chem. 12, 771 (1983).
F. Quirion, D. Lambert, and G. Perron,Can. J. Chem. 70, 2745 (1992).
S. D. Christian,J. Phys. Chem. 64, 764 (1960); H. Mackle and R. T. B. McLean,Trans. Faraday Soc. 56, 115 (1960); P. Pollack and R. T. B. McLean,Can. J. Chem. 45, 3089 (1967); M. R. Mohilner, L. M. Bowman, S. T. Freeland, and H. Nakadomari,J. Electrochem. Soc. 120, 1658 (1973).
J. Biais, L. Adberg, and P. Stenius,J. Colloid Inter. Sci. 86, 350 (1982).
P. Picker, E. Tremblay, and C. Jolicoeur,J. Solution Chem. 3, 6377 (1974).
P. Picker, P.-A. Leduc, P. R. Philip, and J. E. Desnoyers,J. Chem. Thermodyn.,3 631 (1971).
J. P. Cox and E. F. G. Herrington,Trans. Faraday Soc. 52, 928 (1956).
G. P. Gladden and M. M. Breuer,J. Colloid Inter. Sci. 53, 249 (1975).
Complete set of tabular data may be purchased from The Depository of Unpublished Data, CISTI, National Research Council of Canada, Ottawa, Canada, K1A 0S2.
B. E. Conway and L. H. Laliberté inHydrogen-Bonded Solvent Systems, A. K. Covington and P. Jones, eds., (Taylor and Francis Ltd, London, 1968) p. 139.
O. Enea, P. P. Singh, and L. G. Hepler,J. Solution Chem. 6, 719 (1977).
G. Perron, L. Couture, and J. E. Desnoyers,J. Solution Chem. 21, 433 (1992).
A. H. Roux and J. E. Desnoyers,Proc. Ind. Acad. Sci. (Chem. Ser.) 98, 435 (1987).
J. E. Desnoyers and C. Jolicoeur inComprehensive Trearise of Electrochemistry, Vol. 5, B. E. Conway, J. O'M. Bockris, and E. Yeager, eds., (Plenum, New York, 1983).
F. Franks and J. E. Desnoyers, inWater Science Reviews, No. 1, F. Franks, ed., (Cambridge University Press, 1985) p. 171.
J. E. Desnoyers,Pure Appl. Chem. 54, 1469 (1982).
S. W. Benson,J. Am. Chem. Soc. 100, 5640 (1978).
M. Fixman,J. Chem. Phys. 36, 1957 (1962).
V. K. Filippov and G. C. Chernik,Thermochimica Acta 101, 65 (1986).
G. Caron and J. E. Desnoyers,J. colloid Inter. Sci. 119, 141 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perron, G., Quirion, F., Lambert, D. et al. Thermodynamic properties of aqueous organic mixtures near the critical demixing: Cases of 2,6-dimethylpyridine and of 2-isobutoxyethanol. J Solution Chem 22, 107–124 (1993). https://doi.org/10.1007/BF00650678
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00650678