Abstract
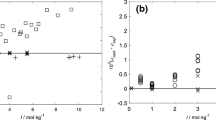
Electromotive-force measurements have been made on HCl−MgCl2−H2O mixtures at 5, 15, 25, 35 and 45°C at eleven different ionic strengths from 0.1–5.0 mol-kg−1. The results are interpreted in terms of the simple Harned's equations, as well as the more complicated Pitzer ion-component treatment of multicomponent electrolyte mixtures. Activity coefficients for HCl in the salt mixtures obey Harned's rule up to and including I=5.0. For the salt in the acid mixtures, Harned's rule holds true up to and including I=0.5. The contribution of higher-order electrostatic terms (Eθ and E'θ) in the Pitzer equations is important for accurate evaluations of unlike cation-cation interactions (θH,Mg), and cation-anion-cation interactions (ΨH,Mg,Cl). The values ofSθH,Mg and ΨH,Mg,Cl (determined with Eθ and E'θ), θH,Mg and ΨH,Mg,Cl (determined without Eθ and E'θ), as well as the trace activity coefficients of HCl, γ Atr , in solutions of MgCl2 (where ionic strength fraction of the salt,y B = 1) at all the experimental temperatures and ionic strengths, are reported. Results of this study are compared with those for similar systems. At I=0.1 and 25°C, the results of the Brönsted-Guggenheim specific interaction theory are discussed briefly.
Similar content being viewed by others
References
R. N. Roy, J. J. Gibbons, J. K. Trower, and G. A. Lee,J. Solution Chem. 9, 535 (1980), and references listed therein.
K. S. Pitzer,J. Phys. Chem. 77, 268 (1973).
K. S. Pitzer and G. Mayorga,J. Phys. Chem. 77, 2300 (1973);J. Solution Chem. 3, 539 (1974).
D. R. White, Jr., R. A. Robinson, and R. G. Bates,ibid.,9, 457 (1980).
K. H. Khoo, C. Y. Chan, and T. K. Lim,ibid. 6, 855 (1977).
M. H. Lietzke and H. A. O'Brien Jr.,J. Phys. Chem. 72, 4408 (1968).
C. Shin and C. M. Criss,J. Chem. Thermodyn. 11, 663 (1979).
R. G. Bates, E. A. Guggenheim, H. S. Harned, D. J. G. Ives, G. J. Janz, C. B. Monk, J. E. Prue, R. A. Robinson, R. H. Stokes, and W. F. K. Wynne-Jones,J. Chem. Phys. 25, 361 (1956);26, 222 (1957).
H. S. Harned and R. A. Robinson,Multicomponent Electrolyte Solutions, 1 st ed., (Pergamon Press, 1968), p. 60.
H. S. Harned and B. B. Owen,The Physical Chemistry of Electrolyte Solutions, 3rd. ed., (Reinhold, New York, 1958), pp. 605, 716.
H. A. C. McKay,Trans. Faraday Soc. 51, 903 (1955).
H. S. Harned,J. Phys. Chem. 63, 1299 (1959);64, 112 (1960);67, 1739 (1963).
M. Sengupta, K. Pal, and A. K. Chakravarti,J. Electroanal. Chem. 79, 19 (1977).
E. A. Guggenheim and J. C. Turgeon,Trans. Faraday Soc. 51, 747 (1955).
C. J. Downes,J. Phys. Chem.,74, 2153 (1970).
K. S. Pitzer and J. J. Kim,J. Amer. Chem. Soc. 96, 5701 (1974).
K. S. Pitzer, R. N. Roy, and L. F. Sylvester,ibid. 99, 4930 (1977).
K. S. Pitzer,J. Solution Chem. 4, 249 (1975).
L. F. Silvester and K. S. Pitzer,ibid. 7, 327 (1978).
H. L. Friedman,Ionic Solution Theory (Interscience, New York, 1962).
C. E. Harvie and J. H. Weare, private communication.
L. F. Silvester and K. S. Pitzer,J. Phys. Chem. 81, 1822 (1977).
H. F. Holmes, C. F. Baes, Jr., and R. E. Mesmer,J. Chem. Thermodyn. 11, 1035 (1978).
K. H. Khoo, T. K. Lim, and C. Y. Chan,J. Solution Chem. 7, 291 (1978) and references listed therein.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roy, R.N., Gibbons, J.J., Bliss, D.P. et al. Activity coefficients for ternary systems: VI. The system HCl + MgCl2 + H2O at different temperatures; application of Pitzer's equations. J Solution Chem 9, 911–930 (1980). https://doi.org/10.1007/BF00646403
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00646403