Abstract
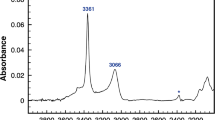
The pleochroic behaviour of two nominally anhydrous structurally similar minerals, danburite and An59 labradorite, was investigated in the region of the OH stretching frequencies. Danburite shows a sharp absorption band at 3540 cm−1, labradorite shows a broad band with an absorption maximum at 3230 cm−1. On the basis of the pleochroic scheme of theinfrared (IR) absorption spectra it is proposed that the OH dipoles in danburite are located within the symmetry plane showing a distinct orientation parallel to [010]; the OH groups in labradorite are oriented approximately perpendicular to (001). The proposed models are in accordance with bond valence calculations showing that in both framework structures the most deficient oxygens, O5 in danburite and O C m in labradorite, are partially replaced by OH.
Similar content being viewed by others
References
Beran A (1976) Messung des Ultrarot-Pleochroismus von Mineralen. XIV. Der Pleochroismus der OH-Streckfrequenz in Diopsid. Tschermaks Mineral Petrogr Mitt 23:79–85
Beran A (1986) A model of water allocation in alkali feldspar, derived from infrared-spectroscopic investigations. Phys Chem Minerals 13:306–310
Beran A, Götzinger MA (1987) The quantitative IR spectroscopic determination of structural OH groups in kyanites. Mineral Petrol 36:41–49
Brown ID, Wu KK (1976) Empirical parameters for calculating cation-oxygen bond valences. Acta Crystallogr B 32:1957–1959
Dunbar C, Machatschki F (1931) Structure of danburite, CaB2Si2O8. Z Kristallogr 76:133–146
Gandais M, Willaime C (1984) Mechanical properties of feldspars. In: Brown WL (ed) Feldspars and feldspathoids, NATO ASI Series C 137. D Reidel Publ Comp, Dordrecht Boston Lancaster, pp 207–246
Griggs D (1967) Hydrolytic weakening of quartz and other silicates. Geophys J R Astron Soc 14:19–31
Hofmeister AM, Rossman GR (1985) A model for the irradiative coloration of smoky feldspar and the inhibiting influence of water. Phys Chem Minerals 12:324–332
Hofmeister AM, Rossman GR (1986) A spectroscopic study of blue radiation coloring in plagioclase. Am Mineral 71:95–98
Lehmann G (1984) Spectroscopy of feldspars. In: Brown WL (ed) Feldspars and feldspathoids, NATO ASI Series C 137. D Reidel Publ Comp, Dordrecht Boston Lancaster, pp 121–162
Machatschki F (1928) Zur Frage der Struktur und Konstitution der Feldspate. Centr Mineral Geol Paläont A/1928:97–104
Martin RF, Donnay G (1972) Hydroxyl in the mantle. Am Mineral 57:554–570
Nakamoto K, Margoshes M, Rundle RE (1955) Stretching frequencies as a function of distances in hydrogen bonds. J Am Chem Soc 77:6480–6486
Phillips MW, Gibbs GV, Ribbe PH (1974) The crystal structure of danburite: a comparison with anorthite, albite, and reedmergnerite. Am Mineral 59:79–85
Ribbe PH (1983) Chemistry, structure and nomenclature of feldspars. In: Ribbe PH (ed) Feldspar mineralogy, Rev Mineral 2. Miner Soc Am, pp 1–19
Ribbe PH (1984) Average structures of alkali and plagioclase feldspars: systematics and applications. In: Brown WL (ed) Feldspars and feldspathoids, NATO ASI Series C 137. D Reidel Publ Comp, Dordrecht Boston Lancaster, pp 1–54
Smith JV (1974) Feldspar minerals, Vol. 1: Crystal structure and physical properties. Springer, Berlin Heidelberg New York
Sugiyama K, Takéuchi Y (1985) Unusual thermal expansion of a B-O bond in the structure of danburite CaB2Si2O8. Z Kristallogr 173:293–304
Taylor WH (1933) The structure of sanidine and other feldspars. Z Kristallogr 85:425–442
Tullis J (1983) Deformation of feldspars. In: Ribbe PH (ed) Feldspar mineralogy, Rev Mineral 2. Miner Soc Am, pp 297–323
Wenk H-R, Joswig W, Tagai T, Korekawa M, Smith BK (1980) The average structure of An 62–66 labroadorite. Am Mineral 65:81–95
Wilkins RWT, Sabine W (1973) Water content of some nominally anhydrous silicates. Am Mineral 58:508–516
Yund RA (1983) Diffusion in feldspars. In: Ribbe PH (ed) Feldspar mineralogy, Rev Mineral 2. Miner Soc Am, pp 203–222
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beran, A. OH groups in nominally anhydrous framework structures: An infrared spectroscopic investigation of danburite and labradorite. Phys Chem Minerals 14, 441–445 (1987). https://doi.org/10.1007/BF00628821
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00628821