Abstract
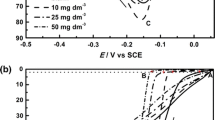
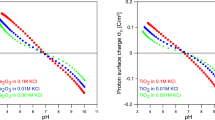
The passivation and dissolution of pure iron in NaOH solutions (1 M–10M) have been studied at 30°, 60° and 80° C by triangular potential sweep voltammetry. The variation of peak height and peak potential with sweep rates for the three anodic peaks in the forward direction and two cathodic peaks in the backward direction when polarized from −1.30 V in 1.0 M NaOH solution, suggest that a monolayer adsorption model holds good. The appearance of limiting currents at higher concentrations of NaOH has been explained in terms of the chemical dissolution of two oxides formed by successive oxidation. The formation of different oxides, hydroxides and solution soluble species under transient conditions has been discussed.
Similar content being viewed by others
References
V. V. Losev and B. N. Kabanov,Zh. Fiz. Khim. 28 (1954) 824.
A. M. Surkhotin and K. M. Kartashov,Corros. Sci. 5 (1965) 393.
T. K. Teplinskaya, N. N. Fedorova and S. A. Rozentsveig,Zh. Fiz. Khim. 38 (1964) 2176.
A. J. Salkind, C. J. Venuto and S. U. Falk,J. Electrochem. Soc. 111 (1964) 493.
H. G. Silver and E. Leaks,ibid. 117 (1970) 5.
L. Ojefers,ibid. 123 (1976) 1691.
A. M. Pritchard and B. J. Mould,Corros. Sci. 11 (1971) 1.
Y. Geronov, I. Tomov and S. Georgiev,J. Appl. Electrochem. 5 (1975) 351.
J. Labat, J. C. Jarrousseatu and J. F. Laurent, Proceedings of Power Sources Conference, Brighton, 1970 (edited by D. H. Coffins), ORIEL Press (1971) pp. 283.
T. Hurlen,Electrochim. Acta 8 (1963) 609.
R. D. Armstrong and I. Baurhoo,J. Electro. anal. Chem. 34 (1972) 41.
R. D. Armstrong and I. Baurhoo,ibid. 40 (1972) 325.
D. S. Poa, J. F. Miller and N. P. Yao, Extended Abstracts, Vol. 83–2, Fall Meeting of Electrochemical Society, Washington, DC, October 9–14, 1983, p. 17.
D. D. Macdonald and D. Owen,J. Electrochem. Soc. 120 (1973) 317.
D. Geana, A. A. El Miligy and W. J. Lorenz,J. Appl. Electrochem. 4 (1974) 337.
R. S. Schrebler Guzman, J. R. Vilche and A. J. Arvia,Electrochim. Acta 24 (1979) 395.
D. D. Macdonald and B. Roberts,ibid. 23 (1978) 781.
V. S. Muralidharan, K. Thangavel and K. S. Rajagopalan,ibid. 28 (1983) 1611.
S. Srinivasan and E. Gileadi,ibid. 11 (1966) 321.
M. Pourbaix, ‘Atlas of Electrochemical Equilibria in Aqueous Solutions’, Pergamon Press, (1966).
A. M. Baticle, R. P. Veerereau and J. Vernieres,J. Electroanal. Chem. 45 (1973) 439.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muralidharan, V.S., Veerashanmugamani, M. Electrochemical behaviour of pure iron in concentrated sodium hydroxide solutions at different temperatures: a triangular potential sweep voltammetric study. J Appl Electrochem 15, 675–683 (1985). https://doi.org/10.1007/BF00620563
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00620563