Summary
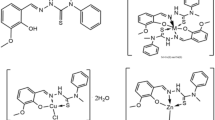
Vanillin thiosemicarbazone (VTSC) has been used to isolate the complexes of the types [M(VTSC)2(H2O)2]X2 (M=MnII, FeII, CoII, or NiII and X=Cl) and [M(VTSC)X2]H2O (M=CuII, ZnII, CdII or HgII and X=Cl). Probable structures of these complexes are suggested on the basis of elemental analysis, molar conductance, magnetic moment and electronic and i.r. spectral data. The fungicidal activity of VTSC and the isolated complexes has been evaluated on pathogenic fungi,Alternaria (Sp.),Paecilomyces (Sp.) andPestalotia (Sp.).
Similar content being viewed by others
References
G. Domagk, R. Behnisch, F. Mietzsch and H. Schmidt,Naturwissenschaften, 33, 315 (1946).
N. N. Orlova, V. A. Aksensova, D. A. Selidovkin, N. S. Bogdanova and G. N. Pershin,Russ. Pharmacol. Toxicol., 348 (1968).
K. Butler,U. S. Pat No. 3,382,266 (1968).
D. J. Bauer, L. S. Vincent, C. H. Kempe and A. W. Downe,Lancet, 2, 494 (1963).
H. G. Petering, H. H. Buskirk and G. E. Underwood,Cancer Res., 64, 367 (1964).
A. Albert and R. J. Goldcre,Nature, 161, 95 (1948).
A. Albert, S. D. Rubbo, R. J. Goldcre and B. G. Balfour,Brit. J. Exptl. Path., 28, 69 (1947).
S. D. Rubbo, A. Albert and N. I. Gibson,Brit. J. Exptl. Path., 31, 425 (1960).
Robbins and McWeigh,Am. J. Bot, 33, 638 (1946).
Y. Okada,Jap. Pat., 7,017,189 (1970);Chem. Abstr., 73, 77252y (1970).
R. S. Srivastava,J. Inorg. Nucl. Chem., 42, 1526 (1980).
P. da Silva Lacaz, M. L. B. Salac, L. M. de Andrade, and I. R. Suassuna,Rev. Brasil Tuberc., 26, 783 (1958).
W. J. Geary,Coord. Chem. Revs., 7, 81 (1971).
K. Nakamoto,Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York (1970).
I. S. Ahuja,J. Inorg. Nucl. Chem., 29, 2091 (1967).
A. Sabatini and I. Bertini,Inorg. Chem., 4, 959 (1965).
D. M. Adams, J. Chatt, J. M. Davidson and J. Gerratt,J. Chem. Soc., 2189 (1963).
W. R. Paryzek,Inorg. Chim. Acta, 93, L43 (1984).
R. S. Srivastava,Inorg. Chim. Acta, 56, L65 (1981).
D. Priest,Viewpoints in Biology, J. D. Carthy and C. L. Duddington, Eds., p. 52. Butterworths, London (1963).
J. C. Bailar, H. J. Emeléus, R. Nyholm and A. F. Trotman-Dickenson,Comprehensive Inorganic Chemistry, vol. 3, p. 3, Pergamon Press, New York (1973).
Author information
Authors and Affiliations
Additional information
On leave from the University of Myosore.
Rights and permissions
About this article
Cite this article
Thimmaiah, K.N., Chandrappa, G.T., Lloyd, W.D. et al. Synthesis and chemical characterization of biologically important complexes of vanillin thiosemicarbazone with manganese(II), iron(II), cobalt(II), nickel(II), copper(II), zinc(II), cadmium(II), and mercury(II). Transition Met Chem 10, 299–302 (1985). https://doi.org/10.1007/BF00619014
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00619014