Abstract
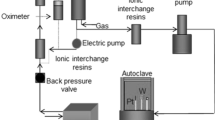
A study has been made of the transpassive dissolution of mild steel in sodium nitrate solution over a range of current densities from 2 to 100 A cm−2. The dissolution current efficiency and the anode potential free from the electrolyte IR component were measured in a flow cell; optical and scanning electron microscopy were then used to examine the sample surfaces after the dissolution tests. The results show that during the early stage of transpassive dissolution, the mild steel is covered with a compact, electronically conductive Fe3O4 film, and the current is consumed mainly in oxygen generation on the film/electrolyte interface. With increasing anode potential and current density, this film is gradually broken and the underlying metal surface becomes exposed to the electrolyte. At this stage, iron dissolution begins at a high rate. The film rupturing process is strongly dependent on nitrate concentration; the higher this is, the lower is the current density required to rupture the film.
Similar content being viewed by others
References
D-T. Chin and A. J. Wallace,J. Electrochem. Soc.,120 (1973) 1487.
D. Landolt,J. Electrochem. Soc.,119 (1972) 708.
D. Landolt, R. H. Muller and C. W. Tobias, inFundamentals of Electrochemical Machining, pp. 200–26, Ed. C. L. Faust, The Electrochemical Society, Inc., Princeton, New Jersey (1971).
D-T. Chin,J. Electrochem. Soc.,119 (1972) 1181.
K-W. Mao,J. Electrochem. Soc.,118 (1971) 1876.
K-W. Mao,J. Electrochem. Soc.,120 (1973) 1056.
K. J. Vetter,Electrochemical Kinetics, Academic Press, New York (1967).
G. J. Janz, B. G. Oliver, G. R. Lakshminaryanan and G. E. Mayer,J. Phys. Chem.,74 (1970) 1285.
A. M. Sukhotin and K. M. Kartashova,Corrosion Sci.,5 (1965) 393.
K-W. Mao, M. A. LaBoda and J. P. Hoare,J. Electrochem. Soc.,119 (1972) 419.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chin, D.T., Mao, K.W. Transpassive dissolution of mild steel in NaNO3 electrolytes. J Appl Electrochem 4, 155–161 (1974). https://doi.org/10.1007/BF00609024
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00609024