Abstract
-
1.
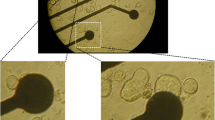
Cells were isolated by incubating chunks of tissue from the urinary bladder of the guinea-pig in a high potassium, low chloride medium containing 0.2 mM calcium plus the enzymes collagenase and pronase. After isolation, the cells were superfused with a physiological salt solution (PSS) containing 150 mM NaCl, 3.6 mM CaCl2 and 5.4 mM KCl (35°C). Patch electrodes filled with an isotonic KCl-solution were used for whole cell recordings. With a single electrode voltage clamp we measured a capacitance of 50±5 pF per cell, an input resistance of 200±25 kOhm ·cm2 and a series resistance of 44±4 Ohm·cm2.
-
2.
The cells had resting potentials of −52±2 mV. They did not beat spontaneously but responded to stimuli with single action potentials (APs) which rose from the threshold (−38 mV) with a maximal rate of 6.5±1.8 V/s to an overshoot of 22±3 mV. The AP lasted for 36±4 ms (measured between threshold and −40 mV). Continuous cathodal current produced repetitive activity, a pacemaker depolarization followed the AP and preceded the next upstroke.
-
3.
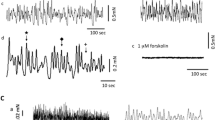
Net membrane currents evoked by clamp steps to positive potentials were composed of an inward and an outward component. The inward component generating the upstroke of the AP was carried by Ca ions (i Ca, Klöckner and Isenberg 1985). The repolarization resulted from a potassium outward currenti K. Ca-channel blockers (5 mM NiCl2) reducedi K suggesting that (part of)i K was Ca-activated.
-
4.
i K rose within about 100 ms to a peak of 40–200 μA/cm2 from which it inactivated slowly and incompletely. The inactivatingi K followed a bell-shaped voltage-dependence, the noninactivatingi K an outwardly rectifying one. Both parts had similar steady state inactivation curves with a half maximal inactivation potential at −36 mV and a slope of 9 mV.
-
5.
Repolarization to −50 mV induced outward tail currents which reversed polarity at −85 mV (the calculated potassium equilibrium potential). The amplitude and the time course of the envelope of the tail currents varied in proportion toi K during the prestep. Thus, the tail current is suggested to reflect the turning off of a potassium conductance which had been activated during the prepulse.
-
6.
i K was largely reduced but not blocked by 20 or 150 mM tetraethylammonium (TEA). TEA did not significantly change the resting potential, but it prolonged the AP and facilitated upstroke and overshoot.i K could be blocked by loading the cells with Cs released from Cs-filled patch electrodes.
-
7.
We compare the results with the data from multicellular tissue (Creed 1971). The more negative resting potential and the absence of spontaneous APs are mainly attributed to the absence of transmitter release from nerve terminals. The isolated cell is suggested as a model of the postsynaptic membrane properties.
Similar content being viewed by others
References
Anderson NC (1969) Voltage-clamp studies on uterine smooth muscle. J Gen Physiol 54:145–165
Anderson GF, Goellner PM, Pierce JM (1972) The electrical properties of isolated detrusor muscle as studied with the sucrose gap. Invest Urol 9:470–475
Bagby RM, Young AM, Dotson RS, Fisher BA, McKinnon K (1971) Contraction of single smooth muscle cells fromBufo marinus stomach. Nature 234:351–352
Bagby RM, Fisher RA (1973) Graded contractions in muscle strips and single cells fromBufo marinus stomach. Am J Physiol 225:105–109
Bendukidze Z, Isenberg G, Klöckner U (1985) Ca tolerant guineapig ventricular myocytes as isolated by pronase in the presence of 250 μM free calcium. Basic Res Cardiol 80:13–18
Benham CD, Bolton TB (1983) Patch-clamp studies of slow potential-sensitive potassium channels in longitudinal smooth muscle cells of rabbit jejumum. J Physiol 340:469–486
Benham CD, Bolton TB, Lang RJ, Takewaki T (1985) The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+-channels in arterial and intestinal smooth muscle cell membranes. Pflügers Arch 403:120–127
Berger W, Grycorcyk R, Schwarz W (1984) Single K+ channels in membrane evaginations of smooth muscle cells. Pflügers Arch 402:18–23
Bolton TB, Tomita T, Vassort G (1981) Voltage clamp and the measurement of ionic conductances in smooth muscle. In: Bülbring E, Brading AF, Jones AW, Tomita T (eds) Smooth muscle, an assessment of current knowledge. Edward Arnold, London, pp 47–64
Connor J, Barr L, Jakobsson E (1975) Electrical characteristics of frog atrial trabeculae in the double sucrose gap. Biophys J 15:1042–1067
Creed KE (1971) Effects of ions and drugs on the smooth muscle cell membrane of the guinea-pig urinary bladder. Pflügers Arch 326:127–141
Creed KE, Ishikawa S, Ito Y (1983) Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol 338:149–164
Dreyer F, Peper K (1974) Iontophoretic application of acetylcholine; advantages of high resistance pipettes in connection with an electronic current pump. Pflügers Arch 348:263–272
Fay FS, Delise CM (1973) Contraction of isolated smooth muscle cells—structural changes. Proc Natl Acad Sci USA 70:641–643
Fay FS, Shlevin HH, Granger WC, Taylor SR (1979) Aequorin luminescence during activation of single isolated smooth muscle cells. Nature 280:506–508
Fisher BA, Bagby RM (1977) Reorientation of myofilaments during contraction of a vertebrate smooth muscle. Am J Physiol 232:C5-C14
Fenwick E, Marty A, Neher E (1982) Sodium and calcium channels in bovine chromaffin cells. J Physiol 331:599–635
Gabella G (1981) Structure of smooth muscles. In: Bülbring E, Brading AF, Jones AW, Tomita T (eds) Smooth muscle. Edward Arnold Ltd, London, pp 1–46
Hamill OP, Marty A, Neher E, Sackmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hermann A, Gorman ALF (1981) Effects of tetraethylammonium on potassium currents in a molluscan neuron. J Gen Physiol 78:87–110
Hermsmeyer K, Mason R (1982) Norepinephrine sensitivity and desensitization of cultured single vascular muscle cells. Circ Res 50:627–632
Hodgkin AL, Huxley AF, Katz B (1952) Measurement of currentvoltage relations in the membrane of the giant axon of Loligo. J Physiol 116:424–448
Inomata H, Kao CY (1976) Ionic currents in the guinea-pigTaenia coli. J Physiol 255:347–378
Imaizumi Y, Watanabe M (1981) The effect of tetraethylammonium chloride on potassium permeability in the smooth muscle cell membrane of canine trachea. J Physiol 316:33–46
Isenberg G (1982) Ca entry and contraction as studied in isolated bovine ventricular myocytes. Z Naturforsch 37c:502–512
Isenberg G, Klöckner U (1980) Glycocalix is not required for slow inward calcium current in isolated rat heart myocytes. Nature 284:358–360
Isenberg G, Klöckner U (1982a) Calcium tolerant ventricular myocytes prepared by preincubation in a “KB-medium”. Pflügers Arch 395:6–18
Isenberg G, Klöckner U (1982b) Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflügers Arch 395:30–41
Johansson B, Somlyo AP (1980) Electrophysiology and excitation-contraction coupling. In: Handbook of physiology, Sect 2/II: Vascular smooth muscle. American Physiological Society, Bethesda, MD, pp 301–323
Johansson B, Mellander S (1975) Static and dynamic changes in vascular myogenic responses to passive changes in length as revealed by electrical and mechanical recordings from the rat portal vein. Circ Res 36:76–83
Johnson EA, Lieberman M (1971) Heart: excitation and contraction. Annu Rev Physiol 33:479–532
Kao CY, McCullough JR (1975) Ionic currents in the uterine smooth muscle. J Physiol 246:1–36
Klöckner U, Isenberg G (1984) Ca-activated potassium currents (i KCa) as studied in smooth muscle cells isolated from the urinary bladder of the guinea-pig. Pflügers Arch 402:R34
Klöckner U, Isenberg G (1985) Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guineapig). Pflügers Arch 405:340–348
Krell RD, McCoy JL, Ridley PT (1981) Pharmacological characterization of the excitatory innervation to the guinea-pig urinary bladder in vitro: evidence for both cholinergic and non-adrenergic non-cholinergic neurotrasmission. Br J Pharmacol 74:15–22
Kurihara S, Creed DE (1972) Changes in the membrane potential of the smooth muscle cells of the guinea-pig urinary bladder in various environments. Jpn J Physiol 22:667–683
Meech RW (1978) Intracellular calcium and the control of membrane permeability. Somp Soc Exp Biol 30:161–191
Mironneau J (1974) Voltage clamp analysis of the ionic currents in uterine smooth muscle using the double sucrose gap method. Pflügers Arch 352:197–210
Mironneau J, Savineau J-P (1980) Effects of calcium ions on outward membrane currents in rat uterine smooth muscle. J Physiol 302:411–425
Momose K, Gomi Y (1980) Studies on isolated smooth muscle cells. vi. Dispersion procedures from acetylcholine-sensitive smooth muscle cells of guinea-pig. Jpn J Smooth Muscle Res 16:29–36
Sibley CNA (1984) A comparison of spontaneous and nervemediated activity in bladder muscle from man, pig and rabbit. J Physiol 354:431–443
Simon W, Amman O, Oehme M, Morf WE (1978) Calcium-selective electrodes. Ann NY Acad Sci 307:52–69
Singer JJ, Walsh JV (1980a) Passive properties of the membrane of single freshly isolated smooth muscle cells. Am J Physiol 239:C153-C161
Singer JJ, Walsh JV (1980b) Rectifying properties of the membrane of single freshly isolated smooth muscle cells. Am J Physiol 239:C175-C181
Singer JJ, Walsh JV (1984) Large conductance Ca2+-activated K+ channels in smooth muscle cell membrane. Reduction in unitary currents due to internal Na+ ions. Biophys J 45:68–70
Thompson SH (1977) Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol 265:465–488
Tsien RW (1983) Calcium channels in excitable membranes. Annu Rev Physiol 45:341–358
Uvelius B (1976) Isometric and isotonic length-tension relations and variations in cell length in longitudinal smooth muscle from rabbit urinary bladder. Acta Physiol Scand 97:1–12
Vassort G (1975) Voltage clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol 252:713–734
Walsh JV, Singer JJ (1980a) Calcium action potentials in single freshly isolated smooth muscle cells. Am J Physiol 239:C162-C174
Walsh JV, Singer JJ (1980b) Penetration-induced hyperpolarization as evidence for Ca2+ activation of K+ conductance in isolated smooth muscle cells. Am J Physiol 239:C182-C189
Walsh JV, Singer JJ (1981) Voltage clamp of single freshly dissociated smooth muscle cells: current-voltage relationships for three currents. Pflüger Arch 390:207–210
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klöckner, U., Isenberg, G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 405, 329–339 (1985). https://doi.org/10.1007/BF00595685
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00595685