Abstract
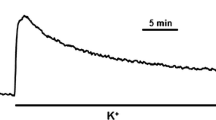
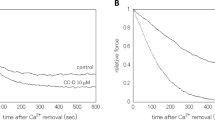
The antipsychotic phenothiazine drugs trifluoperazine, chlorpromazine, and promethazine inhibited Ca2+-activated tension in functionally skinned rabbit ileum and rabbit pulmonary artery strips. Exogenous calmodulin rapidly reversed the inhibition of tension. Inhibition of the endogenous myosin light chain kinase by these drugs resulted with ATP or its analog, ATPγS, as a substrate. The evidence presented suggests the inhibition of Ca2+ activation of tension in skinned smooth muscle preparations by phenothiazines is due to the inhibition of Ca2+-activated phosphorylation of the 20,000 dalton myosin light chain by the endogenous myosin light chain kinase.
Similar content being viewed by others
References
Aksoy MO, Williams D, Sharkey EM, Hartshorne DJ (1976) A relationship between Ca2+ sensitivity and phosphorylation of gizzard actomyosin. Biochem Biophys Res Commun 69:35–41
Brautigan DL, Kerrick WGL, Fischer EH (1980) Insulin and glucose 6-phosphate stimulation of Ca2+ uptake by skinned muscle fibers. Proc Natl Acad Sci USA 77:936–939
Cassidy P, Kerrick WGL (1979) Enzymatic preparation of adenosine 5-O-(3-[35S]thiotriphosphate) by thiophosphorylation of adenosine diphosphate. Biochim Biophys Acta (Amst) 565:209–213
Cassidy P, Hoar PE, Kerrick WGL (1978) Tension development in rabbit ileum smooth muscle skinned by staphylococcal α-toxin: Activation by Ca2+ and Sr2+, irreversible activation in the presence of ATPγS. Biophys J 21:44a
Cassidy P, Hoar PE, Kerrick WGL (1979) Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATPγS. J Biol Chem 254:11148–11153
Cassidy P, Hoar PE, Kerrick WGL (1980) Exogenous calmodulin increases contraction kinetics, calcium ion sensitivity and myosin phosphorylation in smooth muscle. Fed Proc (abstract) 39:2042
Chacko S, Conti MA, Adelstein RS (1977) effect of phosphorylation of smooth muscle myosin in actin activation and Ca2+ regulation. Proc Natl Acad Sci USA 74:129–133
Chaturvedi AK, Landon EJ, Rama Sastry BV (1978) Influence of chlorpromazine on calcium movements and contractile responses of guinea pig ileum longitudinal smooth muscle to agonists. Arch Int Pharmacodyn 236:109–124
Cheung WY (1980) Calmodulin plays a pivotal role in cellular regulation. Science 207:19–27
Dabrowska R, Sherry JMF, Aromatorio DK, Hartshorne DJ (1978) Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry USA 17:253–258
Di Salvo J, Gruenstein E, Silver P (1978) Ca2+ dependent phosphorylation of bovine aortic actomyosin. Proc Soc Exp Biol Med 158:410–414
Eckstein F (1977) Enzymatic synthesis of adenosine 5′-O-(3-[35S]thiotriphosphate). Biochim Biophys Acta (Amst) 483:1–5
Eckstein F (1979) Phosphorothioate analogues of nucleotides. Account Chem Res 12:204–210
Glynn IM, Chappell JB (1964) A simple method for the preparation of32P-labelled adenosine triphosphate of high specific activity. Biochem J 90:147–149
Hellam DC, Podolsky RJ (1969) Force measurements in skinned muscle fibres. J Physiol (Lond) 200:807–819
Hidaka H, Asano M, Iwadare S, Matsumoto I, Totsuka T, Aoki N (1978) A novel vascular relaxing agent, N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, which affects vascular smooth muscle actomyosin. J. Pharm Expt Therap 207:8–15
Hidaka H, Yamaki T, Totsuka T, Asano M (1979) Selective inhibitors of Ca2+-binding modulator of phosphodiesterase produce vascular relaxation and inhibit actin-myosin interaction. Mol Pharmacol 15:49–59
Hoar PE, Kerrick WGL, Cassidy PS (1979) Chicken gizzard: Relation between calcium-activated phosphorylation and contraction. Science 204:503–506
Kerrick WGL, Donaldson SKB (1972) The effects of Mg2+ on submaximum Ca2+-activated tension in skinned fibers of frog skeletal muscle. Biochim Biophys Acta (Amst) 275:117–122
Kerrick WGL, Krasner B (1975) Disruption of the sarcolemma of mammalian skeletal muscle fibers by homogenization. J Appl Physiol 39:1052–1055
Kerrick WGL, Hoar PE, Cassidy PS, Malencik DA (in press) Ca2+ regulation of contraction in skinned muscle fibers. In: Grinnell AD, Brazier MAB (eds) Regulation of muscle contraction: Excitation-contraction coupling. Academic Press, New York
Kobayashi R, Tawata M, Hidaka H (1979) Ca2+ regulated modulator protein interacting agents: Inhibition of Ca2+-Mg2+-ATPase of human erythrocyte ghost. Biochem. Biophys Res Commun 88:1037–1045
Levin RM, Weiss B (1977) Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol 13:690–697
Raess BU, Hinds TR (1979) Plasma membrane Ca2+ transport: Inhibition by trifluoperazine. Pharmacologist 21:227
Seeman P (1977) Anti-schizophrenic drugs. Membrane receptor sites of action. Biochem Pharmacol 26:1741–1748
Sherry JMF, Górecka A, Aksoy MO, Dabrowska R, Hartshorne DJ (1978) Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry USA 17:4411–4418
Sobieszek A (1977) Ca-linked phosphorylation of a light chain of vertebrate smooth-muscle myosin. Eur J Biochem 73:447–483
Swanstrom R, Shank PR (1978) X-ray intensifying screens greatly enhance the detection by autoradiography of the radioactive isotopes32P and125I. Anal Biochem 86:184–192
Walseth TF, Johnson RA (1979) The enzymatic preparation of [γ-32P] nucleoside triphosphates, cyclic [32P]AMP, and cyclic [32P]GMP. Biochim Biophys Acta (Amst) 562:11–31
Weiss B, Levin RM (1978) Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. In: George WJ, Ignarro LJ (eds) Advances in cyclic nucleotide research, Vol 9. Raven Press, New York, pp 285–303
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cassidy, P., Hoar, P.E. & Kerrick, W.G.L. Inhibition of Ca2+-activated tension and myosin light chain phosphorylation in skinned smooth muscle strips by the phenothiazines. Pflugers Arch. 387, 115–120 (1980). https://doi.org/10.1007/BF00584261
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584261