Summary
Serotonin distribution in early Ophryotrocha embryos was investigated with fluorescence microscopy based on formaldehyde gas treatment of the embryos, and with light- and electron-microscopic autoradiography after the embryos had been treated with3H-5-hydroxytryptophan.
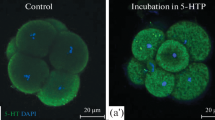
Sections of early cleavage embryos showed serotonin-specific fluorescence all over the blastomeres, but it was mainly concentrated on yolk granules, and to a lesser degree on lipid drops and vacuoles. In 2–8 cell embryos, marked regional concentration of serotonin fluorescence was noticeable along the completed cleavage furrows.
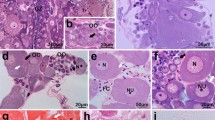
The autoradiographs confirmed the picture of the yolk granules as the principal site of serotonin formation and serotonin accumulation; considerable amounts were also associated with their decomposition products, i.e. lipid drops, vacuoles, and vesicles, whereas major cell organelles, e.g. mitochondria, were almost totally lacking. Of cytoplasmic structures in the blastomeres without apparent yolk granule origin, only microfilaments, particularly those amassed along the cleavage furrow, showed consistent and significant association with formed serotonin. This suggests a connexion between serotonin and microfilaments and might imply that in early embryo cells the fundamental contractile machinery is controlled by serotonin gradually released from the yolk granules.
Within the blastomere nuclei, moderate amounts of serotonin were demonstrated with both fluorescence microscopy and autoradiography.
The monoamine oxidase (MAO) inhibitor catron® (phenylisopropylhydrazine), used to intensify the autoradiographic picture of serotonin in the Ophryotrocha embryos, markedly increased intragranular serotonin accumulation, but also retarded yolk granule disintegration and delayed the cell cleavage process. In embryos barely able to cleave after treatment with catron®, ultrastructural analysis demonstrated that membrane formation at cell cleavage depends on influx of material from the nearby disintegrating yolk granules.
Similar content being viewed by others
References
Björklund, A., Falck, B., Owman, Ch.: Fluorescence microscopic and microspectrofluorimetric techniques for the cellular localization and characterization of biogenic amines. In: The thyroid and biogenic amines (eds. Rall and Kopin), p. 318–368. Amsterdam: North Holland 1972
Bluemink, J. G.: Cytokinesis and cytochalasin-induced furrow regression in the first-cleavage zygote of Xenopus laevis. Z. Zellforsch.121, 102–126 (1971)
Buznikov, G. A., Chudakova, I. V., Zvedzina, N. D.: The role of neurohumours in early embryogenesis. I. Serotonin content of developing embryos of sea urchin and loach. J. Embryol. exp. Morph.12, 563–573 (1964)
Buznikov, G. A., Kost, A. N., Kucherova, N. F., Mndzhoyan, A. L., Suvorov, N. N., Berdysheva, L. V.: The role of neurohumours in early embryogenesis. III. Pharmacological analysis of the role of neurohumours in cleavage divisions. J. Embryol. exp. Morph.23, 549–569 (1970)
Buznikov, G. A., Sakhavova, A. V., Manukhin, B. N., Markova, L. N.: The role of neurohumours in early embryogenesis. IV. Fluorometric and histochemical study of serotonin in cleaving eggs and larvae of sea urchins. J. Embryol. exp. Morph.27, 339–351 (1972)
Deeb, S. S.: Inhibition of cleavage and hatching of sea urchin embryos by serotonin. J. exp. Zool.181, 79–86 (1972)
Emanuelsson, H.: Ultrastructure of nuclei, yolk granules and mitochondria in the early chick blastoderm. Ark. Zool.20, 513–531 (1968)
Emanuelsson, H.: Metabolism and distribution of yolk DNA in embryos of Ophryotrocha labronica LaGreca and Bacci. Z. Zellforsch.113, 450–460 (1971)
Emanuelsson, H.: Karyomeres in early cleavage embryos of Ophryotrocha labronica LaGreca and Bacci. Wilhelm Roux' Archiv173, 27–45 (1973)
Falck, B.: Observations on the possibilities of the cellular localization of monoamines by a fluorescence method. Acta physiol. scand.56, Suppl. 197, 1
Gershon, M. D., Ross, L. L.: Radioisotopic studies of the binding, exchange and distribution of 5-hydroxytryptamine synthesized from its radioactive precursors. J. Physiol. (Lond.)186, 451–476 (1966)
Gershon, M. D., Ross, L. L.: Location of sites of 5-hydroxytryptamine storage and metabolism by radioautography. J. Physiol. (Lond.)186, 477–492 (1966)
Gustafson, T.: Cellular recognition (ed. T. Smith & R. A. Good), p. 47–60. New York: Appleton-Century Crofts 1969
Gustafson, T., Toneby, M.: On the role of serotonin and acetylcholine in sea urchin morphogenesis. Exp. Cell Res.62, 102–117 (1970)
Gustafson, T., Toneby, M.: How genes control morphogenesis. Amer. Sci.59, 452–462 (1971)
Hélène, C., Dimicoli, J.-L., Brun, F.: Binding of tryptamine and 5-hydroxytryptamine (serotonin) to nucleic acids. Fluorescence and proton magnetic resonance studies. Biochemistry (Wash.)10, 3802–3809 (1971)
Humphreys, W. J.: Electron microscope studies of the fertilized egg and the two-cell stage of Mytilus edulis. J. Ultrastruct. Res.10, 244–262 (1964)
Marsland, D. A., Landau, J. V.: The mechanism of cytokinesis: temperature-pressure studies on the cortical gel system in various marine eggs. J. exp. Zool.125, 507–539 (1954)
Mitchison, J. M.: Cell membranes and cell division. Symp. Soc. exp. Biol.6, 105–127 (1952)
Mitchison, J. M.: Microdissection experiments on sea urchin eggs at cleavage. J. exp. Biol.30, 515–524 (1953)
Mitchison, J. M., Cummins, J. E.: The uptake of valine and cytidine by sea urchin embryos and its relation to the cell surface. J. Cell Sci.1, 35–47 (1966)
Mota, M.: Karyokinesis without cytokinesis in the grasshopper. Exp. Cell Res.17, 76–83 (1959)
Quay, W. B.: Comparative physiology of serotonin and melatonin. Advanc. Pharmacol.6A, 283–297 (1968)
Rubin, W., Gershon, M. D., Ross, L. L.: Electron microscope radioautographic identification of serotonin-synthesizing cells in the mouse gastric mucosa. J. Cell Biol.50, 399–415 (1971)
Schroeder, T. E.: The contractile ring. I. Fine structure of dividing mammalian (HeLa) Cells and the effects of cytochalasin B. Z. Zellforsch.109, 431–449 (1970)
Selman, G. G., Perry, M. M.: Ultrastructural changes in the surface layers of the newt's egg in relation to the mechanism of its cleavage. J. Cell Sci.6, 207–227 (1970)
Swann, M. M.: The nucleus in fertilization mitosis and cell division. Symp. Soc. exp. Biol.6, 89–104 (1952)
Szollosi, D.: Cortical cytoplasmic filaments of cleaving eggs: A structural element corresponding to the contractile ring. J. Cell Biol.44, 192–209 (1970)
Thomas, R. J.: Cytokinesis during early development of a teleost embryo: Brachydanio rerio. J. Ultrastruct. Res.24, 232–238 (1968)
tenCate, G.: The formation of enzymes during embryogenesis. In: Proceedings of the Symposium on the Biochemical and Structural Basis of Morphogenesis, p. 108–126. Leiden: E. J. Brill 1952
Tilney, L. G., Marsland, D.: A fine structural analysis of cleavage induction and furrowing in the eggs of Arbacia punctulata. J. Cell Biol.42, 170–184 (1969)
Wessells, N. K., Spooner, B. S., Ash, J. F., Bradley, M. O., Luduena, M. A., Taylor, E. L., Wrenn, J. T., Yamada, K. M.: Microfilaments in cellular and developmental processes. Science171, 135–143 (1971)
Zotin, A. J.: The mechanism of cleavage in amphibian and sturgeon egg. J. Embryol. exp. Morph.12, 247–262 (1964)
Author information
Authors and Affiliations
Additional information
I thank Professor Bengt Falck, Institute of Histology, Lund, for generous provision of facilities that made the fluorescence histoohemical work possible. I thank Dr. Arne Hansson, Malmö, and Dr. Torsten Olsson, Draco Lund, for the gift of various MAO-inhibitors, including catron®. For technical assistance, I am greatly indebted to Mrs Lena Olsson, Mrs Annagreta Petersen, Mrs Gertrud Stridsberg, and Mrs Eva Svensson; and to Mrs Marianne Andersson for typing the manuscript. This work was supported by grants from the Royal Physiographical Society of Lund and the Swedish Natural Science Research Council.
Rights and permissions
About this article
Cite this article
Emanuelsson, H. Localization of serotonin in cleavage embryos of Ophryotrocha labronica La Greca and Bacci. W. Roux' Archiv f. Entwicklungsmechanik 175, 253–271 (1974). https://doi.org/10.1007/BF00574894
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00574894