Abstract
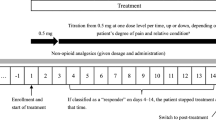
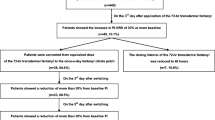
All communications on the use of transdermal fentanyl as well as the recommendations of the manufacturer include the direction that patients should be titrated with a short-acting narcotic to control their cancer pain before they are converted to a fentanyl transdermal therapeutic system (TTS). We investigated the possibility of avoiding this titration phase by immediate fentanyl TTS therapy in patients with uncontrolled cancer pain. Dose finding was performed by direct titration of fentanyl TTS according to clinical necessity on a day-to-day basis. Morphine solution for rescue medication was available. Short-term follow-up was 28 days, and 20 patients (10 in- and 10 outpatients) were evaluable. On the average, sufficient pain control [visual analogue scale (VAS) <35 mm] was reached within 48 h of the start of fentanyl TTS. The mean VAS values before and during fentanyl TTS therapy were 53 mm (before), 27 mm (week 1), 21 mm (week 2), 18 mm (week 3) and 21 mm (week 4). There were statistically significant lower VAS values at all follow-up times compared to pretreatment values (e.g. pretreatment to day 1:P=0.019; pretreatment to day 28:P=0.002, Wilcoxon sign-rank test). The mean fentanyl TTS doses were 70 μg/h (week 1), 98 μg/h (week 2), 107 μg/h (week 3) and 116 μg/h (week 4). The differences of mean fentanyl TTS doses were significantly different between days 1 and 7 (P<0.001) and between days 8 and 14 (P=0.006), but not between days 15 and 21 and days 22 and 28. Mean morphine doses as rescue medication were steadily decreasing from 11 mg/day in week 1 to 3 mg/day in week 4 of treatment, but no statistically significant differences between these amounts could be found. Our results indicate that the titration phase with a short-acting narcotic prior to the conversion to fentanyl TTS is not necessary. Fentanyl TTS can be titrated safely and effectively on a day-to-day basis according to clinical necessity if the patients are well monitored, thus simplifying pain therapy with fentanyl TTS.
Similar content being viewed by others
References
Bailey PL, Stanley TH (1992) Package inserts and other dosage guidelines are especially useful with new analgesics and new analgesic delivery systems. Anaesth Analg 75:873–875
Breda M, Bianchi M, Ripamonti C, Zecca E, Ventafridda V, Paneray AE (1991) Plasma morphine and morphine-6-glucuronide patterns in cancer patients after oral, subcutaneous, sublabial and rectal short-term administration. Int J Clin Pharmacol Res 11:93–97
Glare PA, Walsh TG (1991) Clinical pharmacokinetics of morphine. Ther Drug Monit 13:1–23
Grond S, Zech D, Menser T, Stobbe B, Lehmann KA (1990) Cancer pain relief in dying patients. Pain Suppl 5:S355
Holdiness MR (1989) A review of contact dermatitis associated with transdermal therapeutic systems. Contact Dermatitis 20:3–9
Jage G, Portenoy RK, Foley KM (1990) Die Bestimmung des i.m.-Morphin-Äquivalents zur Therapie des Krebsschmerzes mit verschiedenen Opioiden oder beim Wechsel des Verabreichungsweges. Schmerz 4:110–117
Mather LE, Gourlay GK (1991) Pharmacokinetics of fentanyl. In: Lehmann KA, Zech D (eds) Transdermal fentanyl. Springer, Berlin Heidelberg New York, pp 73–97
Miser AW, Narang PK, Dothage JA, Young RC, Sindelar W, Miser JS (1989) Transdermal fentanyl for pain control in patients with cancer. Pain 37:15–21
Mosser KH (1992) Transdermal fentanyl in cancer pain. Am Fam Physician 45:2289–2294
Portenoy RK, Southam MA, Gupta SK, Lapin J, Layman M, Inturrisi LE, Foley KM (1993) Transdermal fentanyl for cancer pain. Repeated dose pharmacokinetics. Anesthesiology 78:36–43
Schug SA, Zech D, Grond S, Jung H, Menser T, Stobbe B (1992) A longterm survey of morphine in cancer pain patients. J Pain Symptom Manage 7:259–266
World Health Organization (1990) Cancer pain relief and palliative care. WHO, Geneva
Zech D, Grond S, Lynch J (1991) Clinical experience. In: Lehmann KA, Zech D (eds) Transdermal fentanyl. Springer, Berlin Heidelberg New York, pp 171–187
Zech DFJ, Grond SUA, Lynch J, Dauer HG, Stollenwerk B, Lehmann KA (1992) Transdermal fentanyl and initial dose-finding with patient-controlled analgesia in cancer pain. A pilot study with 20 terminally ill cancer patients. Pain 50:293–301
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korte, W., Morant, R. Transdermal fentanyl in uncontrolled cancer pain: titration on a day-to-day basis as a procedure for safe and effective dose finding — a pilot study in 20 patients. Support Care Cancer 2, 123–127 (1994). https://doi.org/10.1007/BF00572094
Issue Date:
DOI: https://doi.org/10.1007/BF00572094