Abstract
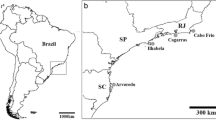
The encrusting spongeHalisarca laxus forms a seemingly obligate association with the stalked solitary ascidianPyura spinifera. In 1991 we examined spatial variation and short-term temporal variation in this association at three neighbouring sites in southeastern Australia. This sponge dominated the surface of almost all the 500 individual ascidians examined, with mean cover usually exceeding 90%. This pattern was consistent among sites and throughout the year of the study. The domination of a small isolated patch of habitable substratum by a sponge is most unusual, given that they are regarded as relatively poor recruiters. To understand how this association might be maintained, we determined the underlying genotypic diversity of the sponge population using starch-gel electrophoresis.P. spinifera is a clump-forming ascidian and usually occurs in clumps of up to 22 individuals. Electrophoretic surveys, based on six variable allozyme loci, revealed that at a total of five plots within three neighbouring New South Wales populations, single sponge genotypes may cover entire ascidian clumps; although a clump sometimes played host to more than one sponge clone. Allele frequencies (averaged across four loci that appear to conform to Mendelian inheritance) showed little variation among populations (standardised genetic variance,F ST=0.013). Nevertheless, sponge populations were genotypically diverse, with samples from 63 of 172 individual clumps displaying unique “clonal” genotypes. Moreover, multi-locus genotypic diversity within all sites approached the level expected for sexual reproduction with random mating. Taken together, these data imply thatH. laxus produces sexually-derived larvae that are at least moderately widelly dispersed. Given the relatively small size of the patches that this sponge inhabits, we also conclude that these larvae are good colonists and good spatial competitors on their ascidian hosts.
Similar content being viewed by others
References
Ayre DJ, Read J, Wishart J (1991) Genetic subdivision within the eastern Australian population of the sea anemoneActinia tenebrosa. Mar Biol 109: 379–390
Battershill CN, Bergquist PR (1990) The influence of storms on asexual reproduction, recruitment and survivorship of sponges. In: Rutzler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington DC, pp 397–403
Bergquist PR (1978) Sponges. University of California Press, Berkeley
Bergquist PR (1995) Dictyoceratida, Dendroceratida and Verongida from the New Caledonian Lagoon (Porifera: Demospongia). Mem Qd Mus 38: 1–51
Curtis ASG, Kerr J, Knowlton N (1982) Graft rejection in sponges. Transplantation 33: 127–133
Davis AR (1996) Association among ascidians: facilitation of recruitment inPyura spinifera. Mar Biol 126: 35–41
Davis AR, White GA (1994) Epibiosis in a guild of sessile subtidal invertebrates in south-eastern Australia: a quantitative survey. J exp mar Biol Ecol 177: 1–14
Grosberg RK (1987) Limited dispersal and proximity dependent mating success in the colonial ascidianBotryllus schlosseri. Evolution 41: 372–384
Harris H, Hopkinson DA (1976) Handbook of electrophoresis in human genetics. North-Holland, Amsterdam
Heyward AJ, Stoddart JA (1985) Genetic structure of two species ofMontipora on a patch reef: conflicting results from electrophoresis and histocompatibility. Mar Biol 85: 117–121
Hidaka M (1985) Tissue compatibility between colonies and between newly settled larvae ofPocillopora damicornis. Coral Reefs 4: 111–116
Hoshino T (1990)Merlia tenuis n. sp. encrusting shell surfaces of gastropods,Chicoreus, from Japan. In: Rutzler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington DC, pp 295–301
Hunt A (1993) Effects of contrasting patterns of larval dispersal on the genetic connectedness of local populations of two intertidal starfish,Patiriella calcar andP. exigua. Mar Ecol Prog Ser 92: 179–186
Hunt A, Ayre DJ (1989) Population structure in the sexually reproducing sea anemoneOulactis muscosa. Mar Biol 102: 537–544
Jackson JBC, Buss L (1975) Allelopathy and spatial competition among coral reef invertebrates. Proc natn Acad Sci USA 72: 5160–5163
Kay AM, Butler AJ (1983) “Stability” of the fouling communities on the pilings of two piers in South Australia. Oecologia 56: 70–78
Keough MJ (1984) Effects of patch size on the abundance of sessile marine invertebrates. Ecology 65: 423–437
Kott P (1985) The Australian Ascidiacea. Part 1. Phlebobranchia and Stolidobranchia. Mem Qd Mus 23: 1–440
Neigel JE, Avise JC (1985) The precision of histocompatibility response in clonal recognition in tropical marine sponges. Evolution 39: 724–732
Pitcher CR, Butler AJ (1987) Predation by asteroids, escape response, and morphometrics of scallops with epizoic sponges. J exp mar Biol Ecol 112: 233–249
Resing JM, Ayre DJ (1985) The usefulness of the tissue grafting bioassay as an indicator of clonal identity in scleractinian corals. Proc 5th int coral Reef Congr 6: 75–81 [Gabrié et al. (eds) Antenne Museum-EPHE, Moorea, French Polynesia]
Richardson BJ, Baverstock PR, Adams M (1986) Allozyme electrophoresis: a handbook for animal systematics and population studies. Academic Press, Sydney
Rinkevich B, Weissman IL (1989) Variation in the outcomes following chimera formation in the colonial tunicateBotryllus schlosseri. Bull mar Sci 45: 213–227
Russ RG (1982) Overgrowth in a marine epifaunal community: competitive hierarchies and competitive networks. Oecologia 53: 12–19
Sebens KP, Thorne BL (1985) Coexistence of clones, clonal diversity, and effects of disturbance. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 357–398
Selander RK, Smith MH, Yang YS, Johnson WB, Gentry JB (1971) Biochemical polymorphism and systematics in the genusPeromyscus. I. Variation in the old-field mouse (Peromyscus polionotus). Stud Genét, Austin, Tex 6: 49–90 (Univ Tex Publ No 7103)
Stoddart JA, Taylor JF (1988) Genotypic diversity: estimation and prediction. Genetics, Austin, Tex 118: 705–711
Swofford D, Selander RB (1981) BIOSYS-1: a FORTRAN program for the comprehensive analysis of electrophoretic data in population genetics and systematics. J Hered 72: 281–283
Wilson CC, Hebert PDN (1992) The maintenance of taxon diversity in an asexual assemblage: an experimental analysis. Ecology 73: 1462–1472
Wright S (1978) Evolution and the genetics of populations. Vol 4. Variability within and among natural populations. University of Chicago Press, Chicago
Author information
Authors and Affiliations
Additional information
Communicated by: G. F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Davis, A.R., Ayre, D.J., Billingham, M.R. et al. The encrusting spongeHalisarca laxus: population genetics and association with the ascidianPyura spinifera . Mar. Biol. 126, 27–33 (1996). https://doi.org/10.1007/BF00571374
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00571374