Abstract
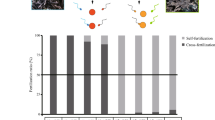
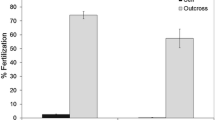
The mating patterns of four species of hermaphroditic scleractinian coral were investigated in November 1984 at Orpheus Island on the Great Barrier Reef, Australia. Each of the species shed eggs and sperm into the water, with subsequent external development of larvae. Studies of gamete viability indicated that cross-fertilizations were possible until at least 6 h after spawning.Montipora digitata cross-fertilized exclusively,Acropora tenuis, Goniastrea aspera andG. favulus were capable of self-fertilization, but to varying extents. In all species, cross-fertilization was the dominant mating pattern.
Similar content being viewed by others
Literature cited
Babcock, R. C.: The biology ofGoniastrea aspera in the Townsville region, 111 pp. Thesis, James Cook University of North Queensland 1980
Babcock, R. C.: Reproduction and distribution of two species ofGoniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs2, 187–195 (1984)
Barnes, H. and M. Barnes: Further observations on self-fertilization inCthamalus sp. Ecology39, p. 500 (1958)
Collins, J. D. and T. A. Walker: A drift card study of the Great Barrier Reef. Final Rep. Gt Barrier Reef mar. Park Auth.Feb., 1985, 1–240 (1985)
Fadlallah, Y. H. and J. H. Pearse: Sexual reproduction in solitary corals: synchronous gametogenesis and broadcast spawning inParacyathus stearnsii. Mar. Biol.71, 233–239 (1982)
Gee, J. M. and G. Brinley Williams: Self-and cross-fertilization inSpirorbis borealis andS. pagenstecheri. J. mar. biol. Ass. U.K.45, 275–285 (1965)
Harriott, V. J.: Reproductive ecology of four scleractinian species at Lizard Island, Great Barrier Reef. Coral Reefs2, 9–18 (1983)
Harrison, P. L., R. C. Babcock, G. D. Bull, J. K. Oliver, C. C. Wallace and B. L. Willis: Mass spawning in tropical reef corals. Science, N.Y.223, 1186–1189 (1984)
Heyward, A. J. and J. D. Collins: Growth and sexual reproduction in the scleractinian coralMontipora digitata (Dana) Aust. J. mar. Freshwat. Res.36, 441–446 (1985a)
Heyward, A. J. and J. D. Collins: Fragmentation inMontipora ramosa: the genet and ramet concept applied to a reef coral. Coral Reefs4, 35–40 (1985b)
Hildemann, W. H., R. L. Raison, G. Cheung, C. J. Hull, L. Akaka and J. Okamoto: Immunological specificity and memory in a scleractinian coral. Nature, Lond.270, 219–223 (1977)
Kojis, B. L. and N.J. Quinn: Aspects of suxual reproduction and larval development in the shallow water hermatypic coralGoniastrea australiensis (Edwards and Haine, 1857). Bull. mar. Sci.31, 558–573 (1981a)
Kojis, B. L. and N. J. Quinn: Reproductive strategies in four species ofPorites (Scleractinia). Manila, Philippines, Proc. 4th int. Symp. coral Reefs2, 145–151 (1981b) (Ed. by E. D. Gomez et al. Quezon City, Philippines: Marine Sciences Center, University of the Philippines)
Kojis, B. L. and N. J. Quinn: Reproductive ecology of two faviid corals (Coelenterata: Scleractinia). Mar. Ecol. Prog. Ser.8, 251–255 (1982)
Morgan, T. H.: The conditions that lead to normal or abnormal development ofCiona. Biol. Bull. mar. biol. Lab., Woods Hole88, 50–62 (1945)
Pianka, H. D.: Ctenophora.In:The reproduction of marine invertebrates, Vol. 1. pp 201–265. Ed. by A. C. Giese and J. S. Pearse. New York: Academic Press. 1974
Reeve, M. and T. C. Cosper: Chaetognatha.In: The reproduction of marine invertebrates, Vol. 2, pp 152–184. Ed. by A. C. Giese and J. S. Pearse. New York: Academic Press 1975
Scofield, V. L., J. M. Schlumberger, L. A. West and I. L. Weissman: Protochordate allorecognition is controlled by an MHC-like gene system. Nature, Lond.295, 499–502 (1982)
Shields, W. M.: Inbreeding and the paradox of sex: a resolution? Evolutionary Theory5, 245–279 (1982)
Stoddart, J. A.: Asexual production of planulae in the coralPocillopora damicornis. Mar. Biol.76, 272–284 (1983)
Stoddart, J. A., D. J. Ayre, B. Willis and A. J. Heyward: Self recognition in sponges and corals? Evolution Lawrence, Kansas39, 461–463 (1985)
Szmant-Froelich, A., P. Yevich and M. E. Q. Pilson: Gametogenesis and early development of the temperate coralAstrangia danae (Anthozoa, Scleractinia). Biol. Bull. mar. biol. Lab., Woods Hole158, 257–269 (1980)
Williams, D. McB., E. Wolanski and J. C. Andrews: Transport mechanisms and potential movements of planktonic larvae in the central region of the Great Barrier Reef. Coral Reefs3, 229–236 (1984)
Author information
Authors and Affiliations
Additional information
Communicated by G. F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Heyward, A.J., Babcock, R.C. Self- and cross-fertilization in scleractinian corals. Mar. Biol. 90, 191–195 (1986). https://doi.org/10.1007/BF00569127
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00569127