Summary
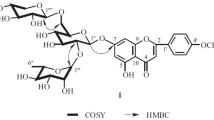
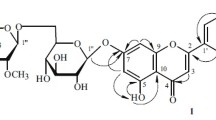
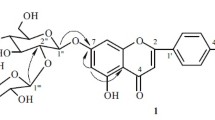
1. The new flavonoid glycoside pasternoside has the structure of isorhamnetin 3-β-D-glucopyranoside 4′-α-L-rhamnopyranoside.
2. The enzymatic hydrolysis of pasternoside has given the monoglycoside deglucopasternoside, which has been shown to be isorhamnetin 4′-α-L-rhamnopyranoside.
Similar content being viewed by others
References
N. P. Maksyutina and D. G. Kolesnikov, DAN SSSR, 142, 1193, 1962.
H. Friedrich, Arch. Pharm., 295, 464, 1962.
M. Ya. Tropp and D. G. Kolesnikov, Med. prom. SSSR, no. 11, 9, 1960.
P. I. Gvozdyak and V. I. Litvinenko, Med. prom. SSSR, no. 5, 20, 1964.
B. V. Chandler and K. A. Harper, Austr. J. Chem., 14, 586, 1961.
V. I. Litvinenko and N. P. Maksyutina, KhPS [Chemistry of Natural Compounds], no. 6, 420, 1965.
I. P. Kovalev and V. I. Litvinenko, KhPS [Chemistry of Natural Compounds], no. 4, 233, 1965.
T. Heap and R. Robinson, J. Chem. Soc., 2336, 1926.
Author information
Authors and Affiliations
Additional information
Khimiya Prirodnykh Soedinenii, Vol. 2, No. 1, pp. 20–26, 1966
Rights and permissions
About this article
Cite this article
Maksyutina, N.P., Litvinenko, V.I. A chemical investigation of pasternoside. Chem Nat Compd 2, 16–20 (1966). https://doi.org/10.1007/BF00566592
Issue Date:
DOI: https://doi.org/10.1007/BF00566592