Summary
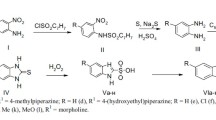
Ten asthmatic patients received an oral dose of3H-ibuterol chloride 3 mg, which upon hydrolysis generates terbutaline equivalent to 2 mg terbutaline sulphate, and3H-terbutaline sulphate 5 mg, in a double-blind, crossover study with randomized administration. After ibuterol the serum concentration of terbutaline rose steeply and then showed a biphasic decline. The maximum level (4.1 ng/ml) of terbutaline occurred after 30 minutes. After terbutaline there was a slow rise and eight patients showed two peaks in the serum curve. The maximum level of terbutaline, 3.0 ng/ml, occurred after 90 minutes. In both cases the average half-life of terbutaline was 3.4 hours. The absorption of ibuterol was twice as great as that of terbutaline. Due to a slight increase in the degree of conjugation after ibuterol ingestion, the bioavailability of the liberated terbutaline was only about 1.6 that of oral terbutaline. Renal clearance data indicated that terbutaline was filtered only via the glomeruli. The metabolic fate of both drugs was similar, as determined by analysis of urine samples. The same three compounds were found: terbutaline and its sulphate conjugate and glucuronide. The mean maximum increase in FEV1.0 was observed 30 minutes after ibuterol and 90 minutes after terbutaline. No significant effect on circulatory parameters was found after either drug. Tremor was noticed by 2 out of 10 patients after ibuterol and by 4 patients after terbutaline. The results of objective measurements of tremor performed with an accelerometer indicated that the amplitude of tremor had to increase to at least twice its basal value, before the patient was aware of it.
Similar content being viewed by others
References
Arner, B.: A comparative clinical trial of different subcutaneous doses of terbutaline and orciprenaline in bronchial asthma. Acta med. scand. Suppl.512 45 - 48 (1970)
Barr, W.H., Riegelman, S.: Intestinal drug absorption and metabolism. I: Comparison of methods and models to study physiological factors ofin vitro andin vivo intestinal absorption. J. pharm. Sci.59 154 - 163 (1970)
Bowman, W.C., Nott, M.W.: Actions of sympathomimetic amines and their antagonists on skeletal muscle. Pharmacol. Rev.21 27 - 72 (1969)
Ciba Guest Symposium: Terminology, definitions and classification of chronic pulmonary emphysema and related conditions. Thorax14 286 - 299 (1959)
Formgren, H.: A clinical comparison of the effect of oral terbutaline and orciprenaline. Scand. J. resp. Dis.51 195 - 202 (1970)
Gaddie, J., Legge, J.S., Palmer, K.N.V.: Aerosols of salbutamol, terbutaline and isoprenaline/phenylephrine in asthma. Brit. J. Dis. Chest67 215 - 220 (1973)
George, C.F., Blackwell, E.W., Davies, D.S.: Metabolism of isoprenaline in the intestine. J. Pharm. Pharmacol.26 265 - 267 (1974)
Harper, N.J.: Drug latentiation. J. med. Pharm. Chem.1 467 - 500 (1959)
Höglund, E., Westling, H., White, T.: Initial human studies with ibuterol, a prodrug to terbutaline. In publication
Kristoffersson, J., Svensson, L.Å., Tegnér, K.: Drug latentiation of terbutaline. Acta pharm. suecica11 427 - 438 (1974)
Lanman, R.C., Gillilan, R., Schanker, L.S.: Absorption of cardiac glycosides from the rat respiratory tract. J. Pharmacol. exp. Ther.187 105 - 111 (1973)
Nilsson, H.T., Persson, C.G.A., Tegnér, K., Ingemarsson, I., Liedberg, G.: Biliary excretion of3H-terbutaline in man. Biochem. Pharmacol.22 3128 - 3129 (1973)
Olsson, O.A.T., Persson, C.G.A., Persson, H., Sörenby, L.: Pharmacological properties of 1-(3′5′-diisobutyryloxy-phenyl)-2-(t-butyl-amino)-ethanol hydrochloride (KWD 2058), a new sympathomimetic bronchodilator. Acta pharmacol. (Kbh.)35 76- 90 (1974)
Persson, K., Persson, K.: The metabolism of terbutalinein vitro by rat and human liver Omethyltransferases and monoamine oxidases. Xenobiotica2 375 -382 (1972)
Sinkula, A.A., Yalkowsky, S.H.: Rationale for design of biologically reversible drug derivatives: Prodrugs. J. pharm. Sci.64 181 - 210 (1975)
Wetterlin, K., Svensson, L.Å.: Swedish Pat. No. 335 359 (1971)
Author information
Authors and Affiliations
Additional information
Subsidiary of AB Astra, Sweden
Rights and permissions
About this article
Cite this article
Hörnblad, Y., Ripe, E., Magnusson, P.O. et al. The metabolism and clinical activity of terbutaline and its prodrug ibuterol. Eur J Clin Pharmacol 10, 9–18 (1976). https://doi.org/10.1007/BF00561543
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00561543