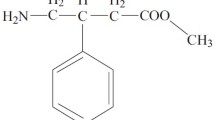
Summary
In vivo criteria are proposed for the assessment of retard formulations. If the pharmacokinetics of the retard formulation tested and of the normal preparation are known, in either plasma or urine, two retard quotients can be defined which define the sustained release. One of the quotients refers to the width (half-value duration) and the other to the height of the plasma concentration time curve of the retard form as compared to the conventional preparation. From these criteria it is possible to draw useful conclusions about thein vivo quality of a retard formulation in an early stage of development of the dosage form. As a practical example the criteria have been applied to Noveril® 240 tablets (dibenzepin hydrochloride), the retard effect of which has made it possible to reduce the frequency of administration from three times daily to once a day.
Similar content being viewed by others
References
Levy, G., Gibaldi, M.: Pharmacokinetics of drug action. Ann. Rev. Pharmacol.12, 85 (1972)
Davies, D. S., Prichard, B. N. C.: Biological effects of drugs in relation to their plasma concentrations. Proceedings of a symposium by the British Pharmacological Society. London: MacMillan 1972
Medgyesi, G., Medgyesi, P.: Bemerkungen zur Pharmakokinetik eines intramuskulär verabreichten Medikaments. Pharmazie22, 253 (1967)
Wagner, J. G.: Biopharmaceutics and Revelant Pharmacokinetics. Drug Intelligence Publications, p. 182. Hamilton, Illinois: Hamilton Press 1971
Unpublished results, Research Institute Wander Ltd., Berne, Switzerland
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meier, J., Nüesch, E. & Schmidt, R. Pharmacokinetic criteria for the evaluation of retard formulations. Eur J Clin Pharmacol 7, 429–432 (1974). https://doi.org/10.1007/BF00560355
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00560355