Abstract
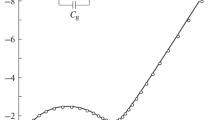
The electrical conductivity (σ) and thermoelectric power (S) of Li3VO4, Li3PO4 and Li3BO3 solidified melts are presented in the temperature range from 415 K to the melting point of each solid. The ionic (σ i,) and electronic (σ e) contributions toσ have been separated over the entire temperature range with the help of a time-dependence study of the d.c. electrical conductivity. Superionic phases in all three solids have been observed below their melting points in which the conductivity is almost purely ionic. The value of the phase transition temperature below which the solid transforms from superionic to normal phase has been obtained. It has been shown that in the normal phase, these solids are mixed conductors. Data for the temperature variations of bothσ i, andσ e are also presented and discussed.
Similar content being viewed by others
References
A. Kvist andA. Lunden,Z. Naturforsch. 20a (1965) 235.
——Idem, ibid. 21a (1966) 1509.
Y. W. Hu, I. D. Raistrick andR. A. Huggins,Mater. Res. Bull. 11 (1976) 1227.
R. A. Huggins,Electrochim. Acta 22 (1977) 773.
R. D. Shanon, B. E. Taylor, A. D. English andT. Berzins, ——ibid. 22 (1977) 783.
R. T. Jonson Jr andR. M. Biefeld, in “Fast Ion Transport in Solids”, edited by P. Vashishta, J. Mundy and S. Shenoy (Eisevier North-Holland, Amsterdam, 1979) p. 457.
H. L. Tuller, D. P. Button andD. R. Uhlman,J. Non-Cryst. Solids 40 (1980) 93.
A. J. Pathak, K. Gaur andH. B. Lal,J. Mater. Sci. Lett. 5 (1986) 785.
——Idem, ibid. 5 (1986) 1058.
K. Gaur, A. J. Pathak andH. B. Lal, ——ibid. 7 (1988) 425.
H. B. Lal, K. Gaur andA. J. Pathak,J. Mater. Sci. in press.
K. Shahi, H. B. Lal andS. Schandra,Indian J. Pure Appl. Phys. 13 (1973) 1.
O. P. Srivastava, PhD thesis, Gorakhpur University (1983).
A. R. West,J. Appl. Electroehem. 3 (1973) 327.
J. Zemann,Acta. Crystallogr. 13 (1960) 863.
F. Stewner, ——ibid. B27 (1971) 904.
I. D. Raistrick andR. A. Huggins, in “Lithium Ion Conducting Solid Electrolytes”, edited by H. V. Venkatasetty (Wiley, New York, 1984) p. 205.
I. T. O. Yukio, M. Katsuki andO. I. Tetsu,J. Non-Cryst. Solids 57 (1983) 389.
M. J. Rice andW. L. Roth,J. Solid. State Chem. 4 (1972) 294.
C. P. Flynn, “Point Defects and Diffusion” (Oxford University Press, London, 1972).
W. J. Pardee andG. D. Mahan,J. Solid State Chem. 15 (1975) 310.
G. D. Mahan,Phys. Rev. B14 (1976) 780.
R. Aronsson, L. Borjesson andL. M. Torell,Phys. Lett. 98A (1983) 205.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gaur, K., Pathak, A.J. & Lal, H.B. Ionic and electronic conductivity in some simple lithium salts. J Mater Sci 23, 4257–4262 (1988). https://doi.org/10.1007/BF00551916
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00551916