Abstract
-
1.
The strain M 5 al of Klebsiella pneumoniae grows excellently with starches. We were able to show that besides the pullulanase associated with the external membrane of the cells the bacterium produces an inducible, extracellular cyclodextrin glucanotrans-ferase [1,4-α-d-glucan-4-α-(1,4-α-glucano)-transferase (cyclising) (EC 2.4.1.19)]. Potato starch and cyclohexaamylose or cycloheptaamylose were found to be the best “inducing” carbon sources for the synthesis of the enzyme. When the bacteria are grown batchwise, maltose is a poorly “inducing” carbon source; larger quantities of the enzyme are synthesized by continuous cultivation with maltose as growth limiting factor.
-
2.
For the determination of the cyclodextrin glucanotransferase-activity an assay method was worked out.
-
3.
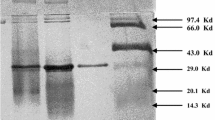
The enzyme could be separated from the culture filtrate and purified to more than 90% in few steps. At a total yield of 61.2% related to the activity of the culture filtrate employed we received an enzyme solution with the specific activity of 26.6 units/mg protein. Some properties of the enzyme are described.
-
4.
The products formed from amylopectin by the enzyme were analyzed. Somewhat more than half the amylopectin was found as cyclodextrins. 29.3% of the cyclodextrin fraction were cycloheptaamylose, 47.2% cyclohexaamylose and 10.7% exo-branched cyclohexaamylose. 12.8% of cyclohexaamylose were obtained from a cyclodextrin glucanotransferase-limit dextrin after debranching by pullulanase and exposing the product to the action of the glucanotransferase again.
-
5.
The importance of the cyclodextrin glucanotransferase for the utilization of starches by this strain of Klebsiella pneumoniae is discussed. After a first characterization the enzyme is compared to the amylase of Bacillus macerans.
Zusammenfassung
-
1.
Der Stamm M 5 al von Klebsiella pneumoniae wächst ausgezeichnet mit Stärken. Wir konnten zeigen, daß das Bacterium neben der mit der externen Membran der Zellen assoziierten Pullulanase eine induzierbare, extracelluläre Cyclodextrin-Glucanotransferase [1,4-α-d-Glucan-4-α-(1,4-α-glucano)-transferase (cyclising) (EC 2.4.1.19)] produziert. Als beste “induzierende” Kohlenstoffquellen erwiesen sich Kartoffelstärke und Cyclohexaamylose bzw. Cycloheptaamylose. Bei statischer Kultivierung ist Maltose eine nur schlecht “induzierende” Kohlenstoffquelle, größere Mengen des Enzyms werden bei kontinuierlicher Züchtung mit diesem Zucker als wachstumslimitierendem Faktor synthetisiert.
-
2.
Für die Bestimmung der Cyclodextrin-Glucanotransferase-Aktivität wurde eine Testmethode ausgearbeitet.
-
3.
Das Enzym konnte in wenigen Schritten aus dem Kulturfiltrat abgetrennt und in guten Ausbeuten zu über 90% rein erhalten werden. Bei einer Gesamtausbeute von 61,2%, bezogen auf die mit dem Kulturfiltrat eingesetzte Aktivität, erhielten wir eine Enzymlösung mit der spezifischen Aktivität von 26,6 E/mg Protein. Einige Eigenschaften des Enzyms werden beschrieben.
-
4.
Die durch das Enzyme aus Amylopectin gebildeten Produkte wurden analysiert. Etwas mehr als die Hälfte des Amylopectins fand sich als Cyclodextrine. 29,3% der Cyclodextrinfraktion waren Cycloheptaamylose, 47,2% Cyclohexaamylose und 10,7% exo-verzweigte Cyclohexaamylose. 12,8% Cyclohexaamylose konnten aus einem Cyclodextrin-Glucanotransferase-Grenzdextrin nach Entzweigung durch Pullulanase und erneuter Einwirkung der Glucanotransferase erhalten werden.
-
5.
Die Bedeutung der Cyclodextrin-Glucanotransferase für die Verwertung von Stärken durch diesen Stamm von Klebsiella pneumoniae wird diskutiert. Nach einer ersten Charakterisierung wird das Enzym mit der amylase von Bacillus macerans verglichen.
Similar content being viewed by others
Literatur
Beisenherz, G., Boltze, H. J., Bücher, T., Czok, R., Garbade, K. H., Meyer-Arendt, E., Pfleiderer, G.: Diphosphofructosealdolase, Phosphoglyceraldehyd-dehydrogenase, Milchsäuredehydrogenase, Glycerophosphat-dehydrogenase und Pyruvatkinase aus Kaninchenmuskulatur in einem Arbeitsgang. Z. Naturforsch. 8b, 555–577 (1953)
Bender, H.: Pullulanase von Aerobacter aerogenes. Lokalisation des zellgebundenen Enzyms kontinuierlich gezüchteter Zellen. Mögliche Assoziation mit dem äußeren Membransystem der Zellen. Arch. Mikrobiol. 71, 331–352 (1970)
Bender, H.: Gewinnung und Trägerfixicrung spezifischer Enzyme zur kontrollierten Modifikation von Polysacchariden. BCT 17, Jahresbericht, Bundesmin. f. Forsch. u. Technol. (1975)
Bender, H., Wallenfels, K.: Untersuchungen an Pullulan. II. Spezifischer Abbau durch ein bakterielles Enzym. Biochem. Z. 334, 79–95 (1961)
Bender, H., Wallenfels, K.: Pullulanase (an amylopectin and glycogen debranching enzyme) from Aerobacter aerogenes In: Methods in enzymology, Vol. VIII (E. F. Neufeld, V. Ginsburg, eds.), pp. 555–559. New York-London: Academic Press 1966
Bernfeld, P.: Amylase, α and β. In: Methods in enzymology, Vol. 1 (S. P. Colowick, N. O. Kaplan, eds.), pp. 149–158. New York-London: Academic Press 1955
Boeltz, F.: 1,4-α-Glucanophosphorylase aus K. pneumoniae M 5 al. Reinigung und Eigenschaften. Diplomarbeit, Universität Freiburg (1975)
Buchanan, R. E., Gibbons, N. E. (eds.): Bergey's Manual of determinative bacteriology. Baltimore, Md.: Williams and Wilkins 1974
Chrambach A., Reisfeld, R. A., Wykoff, M., Zaccari, J.: A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Analyt. Biochem 20, 150–154 (1967)
Drummond, G. S., Smith, E. E., Whelan, W. J.: Mechanism of action of pullulanase. FEBS Lett. 5, 85–88 (1969)
Eisele, B., Rasched, I. R., Wallenfels, K.: Molecular characterization of pullulanase from Aerobacter aerogenes. Europ. J. Biochem. 26, 62–67 (1972)
Essig, U.: Amylomaltase aus K. pneumoniae 1009/III. Diplomarbeit, Universität Freiburg (1976)
French, D.: The Schardinger Dextrins. Advanc. Carbohydr. Chem. 12, 189–260 (1957)
French, D., Levine, M. L., Norberg, E., Nordin, P., Pazur, J. H., Wild, M. G.: Studies on the Schardinger Dextrins. VIII. Cosubstrate specificity in coupling reactions of Macerans-Amylase. J. Amer. chem. Soc. 76, 2387–2390 (1954)
French, D., Pulley, A. D., Effenberger, J. A., Rougvie, M. A., Abdullah, M.: Studies on the Schardinger Dextrins. XII. The molecular size and structure of the δ-, ε-, ζ-, and η-dextrins. Arch. Biochem. Biophys. 111, 153–160 (1965)
Grassmann, W., Hannig, K., Knedel, M.: Electrophoretic determination of serum proteins on filter paper. Dtsch. med. Wschr. 76, 333–336 (1951)
Hernandez, E., Pirt, S. J.: Kinetics of utilization of highly polymerised carbon sources (starch) in a chemostat culture of K. pneumoniae. Pullulanase and α-amylase activities. J. appl. Chem. Biotechnol. 25, 297–304 (1975)
Hope, G. C., Dean, A. C. R.: Pullulanase synthesis in Klebsiella aerogenes strains growing in continuous culture. Biochem. J. 144, 403–411 (1974)
Kainuma, K., Kobayashi, S., Ito, T., Suzuki, S.: Isolation and action pattern of maltohexaose producing amylase from Aerobacter aerogenes. FEBS Lett. 26, 281–285 (1972)
Kainuma, K., Wako, K., Kobayashi, S., Nogami, A., Suzuki, S.: Purification and some properties of a novel maltohexaose-producing exoamylase from Aerobacter aerogenes. Biochim. biophys. Acta (Amst.) 410, 333–346 (1975)
Kitahata, S., Okada, S.: Cyclodextrin glycosyltransferase. II. Action of cyclodextrin transferase from Bacillus megaterium strain No. 5 on starch. Agric. Biol. Chem. 38, 2413–2417 (1974)
Kitahata, S., Tsumyama, N., Okada, S.: Purification and some properties of cyclodextrin glycosyltransferase from a strain of bacillus species. Agric. Biol. Chem. 38, 387–393 (1974)
Knight, J. W.: Modification and uses of wheat starch. In: Starch: Chemistry and technology, Vol. II, Industrial aspects (R. L. Whistler, E. F. Paschall, eds.), pp. 279–291. New York-London: Academic Press 1967
Linder, D., Kurz, G., Bender, H., Wallenfels, K.: 1,4-α-Glucan phosphorylase from K. pneumoniae. Purification and structure. Europ. J. Biochem. (in press, 1976)
Nakamura, N., Horikoshi, K.: Characterization and some cultural conditions of a cyclodextrin glycosyltransferase-producing alkalophilic Bacillus sp. Agr. Biol. Chem. 40, 753–757 (1976a)
Nakamura, N., Horikoshi, K.: Purification and properties of cyclodextrin-glycosyltransferase of an alkalophilic Bacillus. Agr. Biol. Chem. 40, 935–941 (1976b)
Nelson, N.: A photometric adaption of the Somogyi method for the determination of glucose. J. biol. Chem. 153, 375–380 (1944)
Norrman, J., Wöber, G.: Comparative biochemistry of α-glucan utilization in Pseudomonas amyloderamosa and Pseudomonas saccharophila. Physiological significance of variations in the pathways. Arch. Microbiol. 102, 253–260 (1975)
Palmer, T. N., Wöber, G.: The metabolic role of debranching enzymes. Biochem. Soc. Trans. 3, 53–56 (1975)
Palmer, T. N., Wöber, G., Whelan, W. J.: The pathway of exogenous and endogenous carbohydrate utilization in Escherichia coli: A dual function for the enzymes of the maltose operon. Europ. J. Biochem. 39, 601–612 (1973)
Pazur, J. H., Marsh, J. M.: Methyl-α-maltotetraoside from cyclohexaamylose and methyl-α-d-glucopyranoside by Macerans-amylase. In: Methods in carbohydr. chem. Vol. II (R. L.Whistler, M. L. Wolfrom, eds.), pp. 347–349. New York-London: Academic Press 1963
Pollock, M. R.: Exoenzymes. In: The bacteria, a treatise on structure and function, Vol. IV (J. C. Gunsalus, R. Y. Stanier, eds.), pp. 121–178. New York-London: Academic Press 1962
Richard, C.: Etude antigénique et biochimique de 500 souches de Klebsiella. Ann. Biol. clin. 31, 295–303 (1973)
Seitz, J.: Pullulanase aus K. pneumoniae M 5 al. Reinigung und Eigenschaften. Diplomarbeit, Universität Freiburg (1975)
Spiro, R. G.: Analysis of sugars found in glycoproteins. In: Methods in enzymology, Vol. VIII (E. F. Neufeld, V. Ginsburg, eds.), pp. 3–36. New York-London: Academic Press 1966
Thoma, J. A., French, D.: Paper chromatography of homologous saccharides. Selection of solvent components and solvent proportions. Analyt. Chem. 29, 1645–1648 (1957)
Thoma, J. A., Koshland, D. F., Jr.: Competitive inhibition by substrate during enzyme action. Evidence for induced-fit theory. J. Amer. chem. Soc. 82, 3329–3333 (1960)
Thoma, J. A., Stewart, L.: Cycloamyloses. In: Starch: Chemistry and technology, Vol. I, Fundamental aspects (R. L. Whistler, E. F. Paschall, eds.), pp. 209–249. New York-London: Academic Press (1965)
Tilden, E. B., Hudson, C. S.: Preparation and properties of the amylases produced by Bacillus macerans and B. polymyxa. J. Bact. 43, 527–544 (1942)
Trevelyan, W. E., Procter, D. P., Harrison, J. S.: Detection of sugars on paper chromatograms. Nature (Lond.) 166, 444–445 (1950)
Vari-Kutka, M., Bender, H.: Gewinnung und Trägerfixierung spezifischer Enzyme zur kontrollierten Modifikation von Polysacchariden. BCT 17, Jahresbericht, Bundesmin. f. Forsch. u. Technol. (1974)
Viles, F. J., Silverman, L.: Determination of starch and cellulose with anthrone. Analyt. Chem. 21, 950–953 (1949)
Wallenfels, K., Bender, H.: Kristallisierte Pullulanase. Ger. Offen 2 245 342 (Cl. 12d C) (1974)
Wallenfels, K., Bender, H.: Gewinnung und Trägerfixierung spezifischer Enzyme zur kontrollierten Modifikation von Polysacchariden. BCT 17, Jahresbericht, Bundesmin. f. Forsch. u. Technol. (1975)
Wallenfels, K., Bender, H., Vari-Kutka, M.: Gewinnung und Trägerfixierung spezifischer Enzyme zur kontrollierten Modifikation von Polysacchariden. BCT 17, Jahresbericht, Bundesmin. f. Forsch. u. Technol. (1974)
Wöber, G.: The pathway of maltodextrin metabolism in Pseudomonas stutzeri. Hoppe-Seylers Z. physiol. Chem. 354, 75–82 (1973)
Yokobayashi, K., Akai, H., Sugimoto, T., Hirao, M., Sugimoto, K., Harada, T.: Comparison of the kinetic parameters of Pseudomonas isoamylase and Aerobacter pullulanase. Biochim. biophys. Acta (Amst.) 293, 197–202 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bender, H. Cyclodextrin-Glucanotransferase von Klebsiella pneumoniae . Arch. Microbiol. 111, 271–282 (1977). https://doi.org/10.1007/BF00549366
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00549366