Abstract
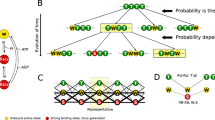
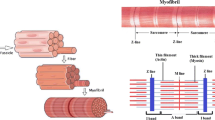
Properties of the rigor state in muscle can be explained by a simple cross-bridge model, of the type which has been suggested for active muscle, in which detachment of cross-bridges by ATP is excluded. Two attached cross-bridge states, with distinct force vs. distortion relationships, are required, in addition to a detached state, but the attached cross-bridge states in rigor muscle appear to differ significantly from the attached cross-bridge states in active muscle. The stability of the rigor force maintained in muscle under isometric conditions does not require exceptional stability of the attached cross-bridges, if the positions in which attachment of cross-bridges is allowed are limited so that the attachment of cross-bridges in positions which have minimum free energy is excluded. This explanation of the stability of the rigor state may also be applicable to the maintenance of stable rigor waves on flagella.
Similar content being viewed by others
References
Cooke R, Thomas D (1980) Spin label studies of the structure and dynamics of glycerinated muscle fibers: application. Fed Proc 39:1962
Eisenberg E, Hill TL (1978) A cross-bridge model of muscle contraction. Prog Biophys Mol Biol 33:55–82
Eisenberg E, Hill TL, Chen Y (1980) Cross-bridge model of muscle contraction; quantitative analysis. Biophys J 29:195–227
Gibbons BH, Gibbons IR (1974) Properties of flagellar “rigor waves” formed by abrupt removal of adenosine triphosphate from actively swimming sea urchin sperm. J Cell Biol 63:970–985
Guth K, Kuhn HJ (1978) Stiffness and tension during and after sudden length changes of glycerinated rabbit psoas muscle fibers. Biophys Struct Mech 4:223–236
Haselgrove J, Huxley HE (1973) X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting muscle. J Mol Biol 77:549–568
Heinl P, Kuhn HJ, Ruegg JC (1974) Tension responses to quick length changes of glycerinated skeletal muscle fibers from the frog and tortoise. J Physiol (Lond) 237:243–258
Hill TL (1974) Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol 28:267–340
Hill TL (1975) Theoretical formalism for the sliding filament model of contraction of striated muscle. Part II. Prog Biophys Mol Biol 29:105–159
Hindmarsh AC (1974) GEAR: Ordinary Differential Equation System Solver, Lawrence Livermore Laboratory Report UCID-30001, Revision 3
Holmes KC, Tregear RT, Barrington Leigh J (1980) Interpretation of the low angle X-ray diffraction from insect flight muscle in rigor. Proc R Soc Lond B207:13–33
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Huxley AF, Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Huxley HE, Brown WJ (1967) The low-angle X-ray diagram of vertebrate striated muscle and its behavior during contraction and rigor. J Mol Biol 30:383–434
Julian FJ, Sollins MR (1972) Regulation of force and speed of shortening in muscle contraction. Cold Spring Harbor Symp Quant Biol 37:635–646
Kuhn HJ (1978a) Cross-bridge slippage induced by the ATP analogue AMP-PNP and stretch in glycerol-extracted fibrillar muscle fibers. Biophys Struct Mech 4:159–168
Kuhn HJ (1978b) Tension transients in fibrillar muscle fibers as affected by stretch-dependent binding of AMP-PNP: a teinochemical effect. Biophys Struct Mech 4:209–222
Kuhn HJ, Güth K, Drexler B, Berberich W, Ruegg JC (1979) Investigation of the temperature dependence of the cross-bridge parameters for attachment, force generation and detachment as deduced from mechano-chemical studies in glycerinated single fibers from the dorsal longitudinal muscle of Lethocerus maximus. Biophys Struct Mech 6:1–29
Lindemann CB, Rudd WG, Rikmenspoel R (1973) The stiffness of the flagella of impaled bull sperm. Biophys J 13:437–448
Maréchal G (1960) Rigidité et plasticité du muscle strié en contracture iodoacétique. Arch Int Physiol Biochim 68:740–760
Miller A, Tregear RT (1972) Structure of insect fibrillar flight muscle in the presence and absence of ATP. J Mol Biol 70:85–104
Mulvany MJ (1975) Mechanical properties of frog skeletal muscles in iodoacetic acid rigor. J Physiol (Lond) 252:319–334
Offer G, Elliott A (1978) Can a myosin molecule bind to two different actin filaments? Nature 271:325–329
Okuno M (1980, in press) Inhibition and relaxation of sea urchin sperm flagella by vanadate. J Cell Biol
Okuno M, Hiramoto Y (1979) Direct measurement of the stiffness of echinoderm sperm flagella. J Exp Biol 79:235–344
Squire JM (1977) The structure of the insect thick filaments. In: Tregar RT (ed) Insect flight muscle. Elsevier/North Holland, Amsterdam, pp 91–112
White DCS (1970) Rigor contraction in glycerinated insect flight and vertebrate muscle. J Physiol (Lond) 208:538–605
White DCS, Thorson J (1973) The kinetics of muscle contraction. Prog Biophys Mol Biol 27:175–255
Yamamoto T, Herzig JW (1978) Series elastic properties of skinned muscle fibers in contraction. Pfluegers Arch 373:21–24
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pate, E.F., Brokaw, C.J. Cross-bridge behavior in rigor muscle. Biophys. Struct. Mechanism 7, 51–63 (1980). https://doi.org/10.1007/BF00538158
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00538158