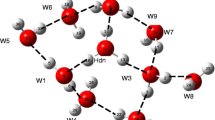
Abstract
Semi-empirical molecular orbital methods proposed up to now seriously fail to describe hydrogen bonded systems associated with (H2O) n . A new scheme of parametrization using a semi-empirical method is proposed. We tested hydrogen bonding associated with the water clusters (H2O) n . The results are found to be close to ab initio Hartree-Fock quality, indicating a good promise for studying hydrogen-bonding systems other than O-H...O moiety.
Similar content being viewed by others
References
Thiel W (1978) Theor Chim Acta 48:357
Gordon MS, Tallman DE, Monroe C, Steinbach M, Armbrust J (1975) J Am Chem Soc 97:1326
Zielenski TJ, Breen DL, Rein R (1978) J Am Chem Soc 100:6266
Klopman G, Andreozzi P, Hopffinger AJ, Kibuchi O, Dewar MJS (1978) J Am Chem Soc 100:6268
Pople JA, Beveridge DL (1970) Approximate molecular orbital theory. McGraw-Hill, New York
Dewar MJS (1967) The molecular orbital theory of organic chemistry. McGraw-Hill, New York
Bingham RC, Dewar MJS, Lo DH (1975) J Am Chem Soc 97:1285
Dewar MJS, Thiel W (1977) J Am Chem Soc 99:4899; (1977) ibid 4907
Dyke TR, Mack KM, Muenter JS (1977) J Chem Phys 15:498
Roothaan CCJ (1951) Rev Mod Phys 23:69; Schaefer III HF (1972) The electronic structure of atoms and molecules. Addison-Wesley, Reading, Massachusetts
Matsuoka O, Clementi E, Yoshimine M (1976) J Chem Phys 64:1351
Morokuma K, Pederson L (1968) J Chem Phys 48:3275
Morokuma K, Winich JR (1970) J Chem Phys 52:1301
Hankins D, Moskowitz JW, Stillinger FH (1970) J Chem Phys 53:4544
Del Bene J, Pople JA (1970) J Chem Phys 52:4858
Popkie H, Kistenmacher H, Clementi E (1973) J Chem Phys 58:5296
Diercksen GHF (1971) Theoret Chim Acta 21:335, (1975) 26:249
Kollman PA, Allen LC (1969) J Chem Phys 51:3286
Curtiss LA, Pople JA (1975) J Mol Spectrosc 55:1
Curtiss LA, Frurip DJ, Blander M (1979) J Chem Phys 71:2703
Eisenberg D, Kauzmann W (1969) The structure and properties of water. Oxford University Press, New York
Jeziorski B, Van Hemert M (1976) Mol Phys 31:713
Clementi E (1976) Lecture Notes in Chemistry 2. Springer, Berlin Heidelberg New York
Suck Salk SH, Kassner JL, Yamaguchi Y (1979) Appl Opt 18:2609; Suck Salk SH, Wetmore AE, Chen TS, Kassner JL (1982) ibid 21:1610
Young V, Suck Salk SH, Hellmuth EW (1979) J Appl Phys 50:6088
Dewar MJ, Yamaguchi Y, Suck Salk SH (1978) Chem Phys Lett 59:541; Dewar MJS, Yamaguchi Y, Draiswamy S, Sharma SD, Suck Salk SH (1979) Chem Phys 41:21; Dewar MJS, Yamaguchi Y, Suck Salk SH, unpublished work; Dewar MJS, Yamaguchi Y, Suck Salk SH (1979) Chem Phys 43:145
Owicki JC, Shipman LL, Scheraga HA (1975) J Phys Chem 79:1794
Tomoda S, Kimura K (1983) Chem Phys Lett 102:560
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) J Am Chem Soc 107:3902
Burstein KY, Isaev AN (1984) Theor Chim Acta 64:397
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salk, S.H.S., Chen, T.S., Hagen, D.E. et al. A study of hydrogen bond strengths of neutral water clusters (H2O) n using modified MNDO. Theoret. Chim. Acta 70, 3–10 (1986). https://doi.org/10.1007/BF00531146
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00531146