Abstract
The vacuum ultraviolet absorption spectra of styrene, benzaldehyde, acetophenone and benzonitrile have been measured in the wavelength region of 1500 to 2200 Å. The absorption bands in the vacuum ultraviolet region appear at 200, 196, 189, 180 and 163 mμ for styrene; at 195,186, 178 and 165 mμ for benzaldehyde; at 196, 191, 179 and 167 mμ for acetophenone; at 187.5, 180 and 167 mμ for benzonitrile.
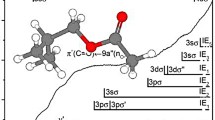
Theoretical studies of π-electron structures have been carried out with styrene, benzal-dehyde and acetophenone by considering configurational interactions among the ground, locally excited, and charge-transfer configurations. The calculated transition energies and oscillator strengths are in good agreement with the observed values. The theoretical results show that the contribution of the charge-transfer configuration amounts to 58 and 68% in the excited states of the 238 mμ band of styrene and of the 232 mμ band of benzaldehyde respectively. This means that these two bands may be regarded as the intramolecular charge-transfer band.
The electron affinity of the C=C and C=O groups were determined to be respectively −0.84 and −1.20 eV from the present analysis of the ultraviolet spectra.
Zusammenfassung
Die Vakuum-Ultraviolettspektren von Styrol, Benzaldehyd, Acetophenon und Benzoni-tril im Bereich von 1500 bis 2200 Å wurden gemessen: 200, 196, 189, 180 und 163 mμ für Styrol, 195,186,178 und 165 mμ für Benzaldehyd, 196, 191, 179 und 167 mμ für Acetophenon und 187,5, 180 und 167 mμ für Benzonitril im Vakuum-UV-Bereich.
Berechnungen der π-Elektronensysteme der drei erstgenannten Verbindungen wurden unter Einschluß von Konfigurationen mit und ohne Charge Transfer durchgeführt und ergaben Übergangsenergien und Oszillatorstärken, die mit den Meßwerten befriedigend übereinstim-men. Zum oberen Zustand der 238 mμ-Bande von Styrol trägt die Charge Transfer Konfiguration 58% bei, und zu dem der 232 mμ-Bande von Benzaldehyd sogar 68%, woraus hervorgeht, daß es sich bei diesen beiden Banden um intramolekulare Charge Transfer Banden handelt.
Die Elektronenaffinität der C=C- und der C=O-Gruppe beträgt nach diesen Berech-nungen −0,84, bzw. −1,20 eV.
Résumé
Nous avons mesuré les spectres d'absorption du styrène, du benzaldehyde, de l'acéto-phénone et du benzonitrile entre 1500 et 2200 Å. Les bandes se situent à 200, 196, 89, 180 et 163 mπ pour le styrène; à 195, 186, 178 et 165 mπ pour le benzaldéhyde; à 196, 191, 179 et 167 mπ pour l'acétophénone et à 187,5, 180 et 167 mπ pour le bentonitrile.
Les structures π-électroniques des trois premières molécules ont été étudiées, en considé-rant l'interaction entre les configurations forndamentales, localement excitées et excitées avec transfert de charge. Les énergies et forces oscillatrices calculées s'accordent bien aux valeurs observées. Le calcul montre que la configuration à transfert de charge entre avec 58% dans la bande à 238 mπ du styrène, et avec 68% dans celle à 232 mπ du benzaldéhyde. Par conséquent ces deux bandes peuvent être attribuées au transfert de charge intramoléculaire. Notre analyse des spectres donne les affinités électroniques des groupes C=C et C=O à −0,84 et −1,20 eV, respectivement.
Similar content being viewed by others
References
Bloor, J. E., and F. Peradejordi: Theoret. chim. Acta (Berl.) 1, 83 (1962).
Kimura, K., H. Tsubomura, and S. Nagakura: Bull. chem. Soc. Japan 37, 1336 (1964).
-, and S. Nagakura: Mol. Physics. (In the press).
Klevens, H. P., and J. R. Platt: Technical Report of the Laboratory of Molecular Structure and Spectra, Department of Physics, University of Chicago, 1954–55.
Longuet-Higgins, H. C., and J. N. Murrell: Proc. phys. Soc. London A 68, 601 (1955).
Mulliken, R. S., C. A. Rieke, D. Orloff, and H. Orloff: J. chem. Physics, 17, 1248 (1949).
Nagakura, S.: Mol. Physics 3, 105 (1960).
—, M. Kojima, and Y. Maruyama: J. Molec. Spect. 13, 174 (1964).
Pariser, R., and R. G. Parr: J. chem. Physics 21, 767 (1953).
Peacock, T. E., and P. T. Wilkinson: Proc. phys. Soc. 79, 105 (1962).
Platt, J. R., H. B. Klevens, and W. C. Price: J. chem. Physics 17, 466 (1949).
Tanaka, J.: J. chem. Soc. Japan (Nippon Kagaku Zasshi) 79, 94 (1958).
Tsubomura, H., K. Kimura, K. Kaya, J. Tanaka, and S. Nagakura: Bull. chem. Soc. Japan 37, 417 (1964).
Walsh, A. D.: Trans. Faraday Soc. 42, 62 (1946). A. D. Walsh: Proc. Roy. Soc. (London) A 191, 32 (1947).
—: Trans. Faraday Soc. 42, 66 (1946).
Watanabe, K.: J. chem. Physics 26, 542 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kimura, K., Nagakura, S. Vacuum ultraviolet spectra of styrene, benzaldehyde, acetophenone, and benzonitrile. Theoret. Chim. Acta 3, 164–173 (1965). https://doi.org/10.1007/BF00527348
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00527348