Abstract
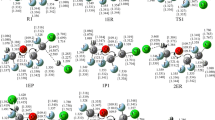
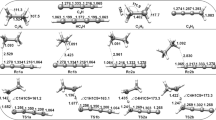
The influence of the solvent on the activation energy of the synchronous, concerted, narcissistic reaction: CH3F + F−→¦CH3F2¦−→FCH3+F−, has been investigated by means of CNDO/2 method. The reaction path has been fully followed in vacuo, and at a suitable point only, of reaction coordinate, in solution. The geometries of H2O, F−, CH3F, and ¦CH3F2¦−, in vacuo and in different hydrated cages, have been optimized.
Zusammenfassung
Der Einfluß des Lösungsmittels auf die Aktivierungsenergie der synchronverlaufenden Reaktionen CH3F+F−→¦CH3F2¦→FCH3+ F−, wurde mit der CNDO/2 Methode geprüft. Der Reaktions-Ablauf wurde in Vakuum vollkommen und in der Lösung an einem geeigneten Punkt der Reaktionskoordinate durchgeführt. Die Geometrien von H2O, F−, CH3F, und ¦CH3F2¦− im Vakuum und in verschiedenen hydrierten Käfigen wurden optimalisiert.
Résumé
L'influence du solvent sur l'énergie d'activation de la réaction synchronique, concertée, narcissistique: CH3F+ F−→¦CH3F2¦−→FCH3+F− a été examinée par la méthode CNDO/2. On a suivie l'entier chemin de réaction in vacuo, alors qu'on a observé un seul point convenable de la coordonée de réaction en solution. On a optimize les geometries de H2O, F−, CH3F, et ¦CH3F2¦− in vacuo et en differentes cages hydratés.
Similar content being viewed by others
References
Ritchie,C.D., King,H.F.: J. Amer. chem. Soc. 88, 1069 (1966).
— —: J. Amer. chem. Soc. 90, 825 (1968).
— —: J. Amer. chem. Soc. 90, 833 (1968).
— —: J. Amer. chem. Soc. 90, 838 (1968).
Lowe,J.P.: J. Amer. chem. Soc. 93, 301 (1971).
Salem,L., Durup,J., Bergeron,G., Cazes,D., Chapuisat,X., Kagan,H.: J. Amer. chem. Soc. 92, 4472 (1970).
Dedieu,A., Veillard,A.: Chem. Physics Letters 5, 328 (1970).
Bertier,G., David,D.I., Veillard,A.: Theoret. chim. Acta (Berl.) 14, 329 (1969).
Pople,J.A., Santry,D.P., Segal,G.A.: J. chem. Physics 43, S 129 (1965).
—, Segal,G.A.: J. chem. Physics 43, S 136 (1965).
— —: J. chem. Physics 44, 3289 (1966).
Segal,G.A.: CNDO/2, molecular calculations with complete neglecte of differential overlap, Program 91, Quantum Chemistry Program Exchange, Indiana University (1966).
Burton,R.E., Daly,J.: Trans. Faraday Soc. 66, 1281 (1970).
Del Bene,J., Pople,J.A.: Chem. Physics Letters 4, 426 (1969).
Tables of interatomic distances and configurations in molecules and ions. London: Spec. Publ. n∘ 11, Scient. Ed. Button. The Chemical Society (1958), M 37 s.
ibidem, M 112.
Pimentel,G.C., McClellan,A.L.: The hydrogen bond, p. 260. San Francisco: W.H. Freeman and Co., 1960.
Pedersen,L.: Chem. Physics Letters 4, 280 (1969).
Hougen,O.A., Watson,K.M., Ragatz,R.A.: Chemical process principles, Part. I, p. 279. New York: John Wiley and Sons, Inc., 1954.
Morris,D.F.C.: Structure and bonding, Vol. IV, p. 63. Berlin: Springer 1968. (Eds. C. K. Jørgensen, J. B. Neilands, R. S. Nyholm, D. Reineu, R. J. P. Williams).
Lischka,H., Plesser,Th., Schuster,P.: Chem. Physics Letters 6, 263 (1970).
Glew,D.N., Moelwyn-Hughes,E.A.: Discuss. Faraday Soc. 15, 150 (1953).
Duke, A.J., Bader, R.F.W.: Chem. Physics Letters 10, 631 (1971).
Author information
Authors and Affiliations
Additional information
Financial aid from Italian “Centro Nazionale delle Ricerche” is gratefully acknowledged.
Rights and permissions
About this article
Cite this article
Cremaschi, P., Gamba, A. & Simonetta, M. The influence of solvation on the calculated activation energy for the reaction CH3F+F− . Theoret. Chim. Acta 25, 237–247 (1972). https://doi.org/10.1007/BF00527290
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00527290