Summary
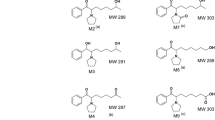
Racemic methtryptoline (1-methyltetrahydro-β-carboline) and 5-hydroxymethtryptoline-9-carboxylic acid (6-hydroxy-1-methyltetrahydro-β-carboline-1-carboxylic acid) were administered intraperitoneally to rats and the components of their urine was subsequently investigated by chiral gas chromatography-mass spectrometry. Methtryptoline rapidly became hydroxylated in the 5- and 6-position and excreted in urine. There was about a ninefold predominance of the S(−) enantiomer over the other in the 5-hydroxylated species, while the 6-hydroxylation produced a small excess of the R(+) enantiomer. About 75% of the injected dose of methtryptoline was recovered in the urine as 5- and 6-hydroxylated compounds during the first 24 h period, demonstrating that hydroxylation represents the major metabolic pathway. Treatment with 6-hydroxymethtryptoline-9-carboxylic acid led to a fivefold increase in the urinary excretion of 5-hydroxymethtryptoline during the first 24 h period with a predominance of the S(−)-enantiomer, indicating a much smaller conversion rate than from methtryptoline. It was concluded that hydroxylation of methtryptoline is a likely pathway for the natural formation of 5-hydroxymethtryptoline.
Similar content being viewed by others
References
Agurell S, Holmstedt B, Lindgren J-E (1969) Metabolism of 5-methoxy-N,N-dimethyl-tryptamine-14C in the rat. Biochem Pharmacol 18:2771–2781
Airaksinen MM, Kari I (1981) β-Carbolines, psychoactive compounds in the mammalian body. Med Biol 59:21–34
Akibori S, Saito J (1930) Synthese von Harman and Harmin. Ber 63:2245–2248
Akimoto H, Okamura K, Yui M, Shioiri T, Kuramoto M, Kikugawa Y, Yamada S (1974) A new approach to the biogenetictype, asymmetric synthesis of indole and isoquinoline alkaloids by 1,3-transfer of asymmetry. Chem Pharmacol Bull 22:2614–2623
Allen JRF, Beck O, Borg S, Skröder R (1980) Analysis of 1-methyl-1,2,3,4-tetrahydro-β-carboline in human urine and cerebrospinal fluid by gas chromatography-mass spectrometry. Eur J Mass Spectrom 1:171–177
Allen JRF, Holmstedt BR (1980) The simple β-carboline alkaloids. Phytochemistry 19:1573–1582
Ayer WA, Browne LM (1970) Alkaloids of Shepherdia argentea and Shepherdia canadensis. Can J Chem 48:1980–1984
Beck O, Lundman A (1983), Occurrence of 6-hydroxy-1-methyl-1,2,3,4-tetrahydro-β-carboline in tissues and body fluids of rat. Biochem Pharmacol 32:1507–1510
Beck O, Faull KF (1985) Concentrations of the enantiomers of 5-hydroxymethtryptoline in mammalian urine: implications for in vivo biosynthesis. Biochem Pharmacol (in press)
Beck O, Bosin TR, Lundman A, Borg S (1982) Identification and measurement of 6-hydroxy-1-methyl-1,2,3,4-tetrahydro-β-carboline by gas chromatography-mass spectrometry. Biochem Pharmacol 31:2517–2521
Beck O, Faull KF, Barchas JD, Johnson JV, Yost RA (1985) Chiral analysis of urinary 5-hydroxymethtryptoline: implications for endogenous biosynthesis and formation during ethanol intoxication. In: Collins MA (ed) Aldehyde adducts in alcoholism. Alan R Liss Inc, New York, pp 145–160
Bosin TR (1978) Serotonin metabolism. In: Essman WB (ed) Serotonin in health and disease, vol 1. Spectrum Publ, New York, pp 181–300
Buckholtz NS (1980) Neurobiology of tetrahydro-β-carbolines. Life Sci 27:893–903
Eriksson CJP, Dietrich RA (1980) Evidence against a biphasic effect of acetaldehyde on voluntary ethanol consumption in rats. Pharmacol Biochem Behav 13 (Suppl 1):191–296
Greiner B, Rommelspacher H (1984) New metabolic pathways of tetrahydronorharmane (tetrahydro-β-carboline) in rats. Naunyn-Schmiedeberg's Arch Pharmacol 325:349–355
Ho BT, Taylor D, Walker KE, McIsaac W (1972) Metabolism of 6-methoxytetrahydro-β-carboline in rats. Xenobiotica 2:349–362
Holman RB, Elliott GR, Faull F, Barchas JD (1980) Tryptolines: The role of indoleamine-aldehyde condensation products in the effects of alcohol. In: Sander M (ed) Psychopharmacology of alcohol. Raven Press, New York, pp 155–169
Jepson JB, Zaltzman P, Udenfriend S (1962) Microsomal hydroxylation of tryptamine, indoleacetic acid and related compounds, to 6-hydroxy derivatives. Biochim Biophys Acta 62:91–102
Johnson JV, Yost RA, Beck O, Faull KF (1985) The use of tandem mass spectrometry for the identification and quantitation of tryptolines (tetrahydro-β-carbolines) in tissue extracts. In: Collins MA (ed) Acetaldehyd adducts in alcoholism. Alan R Liss Inc., New York, pp 145–160
Kopin IJ, Pare CMB, Axelrod J, Weissbach H (1961) The fate of melatonin in animals. J Biol Chem 236:3072–3075
Kveder S, McIsaac WM (1961) The metabolism of melatonin (N-acetyl-5-methoxytryptamine) and 5-methoxytryptamine. J Biol Chem 236:3214–3220
Lemberger L, Axelrod J, Kopin IJ (1971) The disposition and metabolism of tryptamine and the in vivo formation of 6-hydroxytryptamine in the rabbit. J Pharmacol Exp Ther 177:169–176
Le Men J, Fan C (1959) Separation des stéréoisomères des esters éthyliques des acides py-tétrahydro-nor-harmane 5-carboxylique. Bull Soc Chim France 1866–1871
Repke DB, Ferguson WJ (1982) Synthesis of 5-methoxy- and 5-hydroxy-1,2,3,4-tetrahydro-9H-pyrido(3,4-b) in doles. J Heterocyclic Chem 19:845–848
Shaw GJ, Wright GI, Milne GWA (1976) The synthesis of α,α,β,β-d4-serotonin. Biomed Mass Spectrom 3:146–148
Slotkin T, DiStefano V (1970) Urinary metabolites of harmine in the rat and their inhibition of monoamine oxidase. Biochem Pharmacol 19:125–131
Spenser ID (1959) A synthesis of harmaline. Can J Chem 37:1851–1858
Szara S, Hearst E (1962) The 6-hydroxylation of tryptamine derivatives: a way of producing psychoactive metabolites. Ann NY Acad Sci 96:134–141
Taborsky RG, McIsaac WM (1964) The synthesis and preliminary pharmacology of some 9H-pyrido(3,4-b)indoles (β-carbolines) and tryptamines related to serotonin and melatonin. J Med Chem 7:135–141
Ung-Chhun N, Cheng BY, Pronger DA, Serrano P, Chavez B, Perez RF, Morales J, Collins MA (1985) Alkaloid adducts in human brain: coexistence of 1-carboxylated and non-carboxylated isoquinolines and β-carbolines in alcoholics and nonalcoholics. In: Collins MA (ed) Aldehyde adducts in alcoholism. Alan R. Liss Inc, New York, pp 125–136
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beck, O., Faull, K.F. & Repke, D.B. Rapid hydroxylation of methtryptoline (1-methyltetrahydro-β-carboline) in rat: Identification of metabolites by chiral gas chromatography-mass spectrometry. Naunyn-Schmiedeberg's Arch. Pharmacol. 333, 307–312 (1986). https://doi.org/10.1007/BF00512946
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00512946