Summary
-
1.
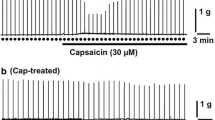
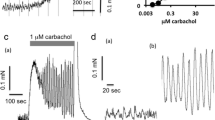
The concentration-effect relationships of adrenergic agonists in inhibiting muscular tone, carbachol-induced contraction of circular muscle strips and nerve-mediated motor activity during the peristaltic reflex have been studied in intact and sympathetically denervated preparations of isolated guinea-pig colon.
-
2.
The order of potencies of adrenergic agonists was different for muscular and nerve-mediated effects, being clonidine > noradrenaline > methoxamine > isoprenaline for the inhibition of peristalsis and isoprenaline > noradrenaline > methoxamine > clonidine for the relaxation of circular muscle.
-
3.
Denervation supersensitivity was specific for the adrenergic agonists and developed both to the muscular and nerve-mediated effects, involving both α and β receptors. The degree of potentiation was similar for noradrenaline and isoprenaline when measured for the muscular effects but was significantly higher for noradrenaline than for isoprenaline or methoxamine when measured for peristalsis inhibition. No potentiation could be observed for papaverine and for the muscular effects of methoxamine and phenylephrine. The increase in potency of noradrenaline ranged from a 26-fold increase for the inhibition of propulsion velocity to a 2.5-fold increase for the inhibition of carbachol-induced contraction. A much narrower range was observed for isoprenaline. Potentiation could also be observed for the inhibitory effect of noradrenaline on acetylcholine release.
-
4.
Clonidine was the most potent agonist against peristaltic reflex and the weakest agonist in relaxing circular muscle. Denervated preparations became subsensitive to the inhibitory effect of clonidine on peristaltic reflex. The potency of clonidine relative to noradrenaline was 488 in intact preparations and only 3.1 in denervated organs.
-
5.
Our results are consistent with a main role played by β receptors in the relaxation of circular smooth muscle and with a selective involvement of α2 receptors in peristalsis inhibition. The pattern of sensitivity changes is consistent with a high degree of physiological modulation by sympathetic supply both on the smooth muscle and on the intramural nervous structures. Both pre- and postjunctional mechanisms could be responsible for the development of supersensitivity, but the former seem to operate only at interneuronal synapses. The opposite sensitivity changes observed for noradrenaline and clonidine suggest a different effect of two agonists at the presynaptic level.
Similar content being viewed by others
References
Bennett A, Stockley HL (1975) The intrinsic innervation of the human alimentary tract and its relation to function. Gut 16:443–453
Bowman WC, Hall TM (1970) Inhibition of rabbit intestine mediated by α- and β-adrenoceptive receptors. Br J Pharmacol 38:399–415
Costa M, Furness JB (1976) The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn-Schmiedeberg's Arch Pharmacol 294:47–60
Costa M, Furness JB (1982) Nervous control of intestinal motility. In: Bertaccini G (ed) Mediators and drugs in gastrointestinal motility. Handbook of experimental pharmacology, vol 59/1. Springer, Berlin Heidelberg New York, pp 279–382
Docherty JR (1983) An investigation of presynaptic α-adrenoceptor subtypes in the pithed rat heart. Br J Pharmacol 78:655–657
Drew GM (1978) Pharmacological characterization of the presynaptic α-adrenoceptors regulating cholinergic activity in the guinea-pig ileum. Br J Pharmacol 64:293–300
Ekström J (1979) Supersensitivity of the rat urinary bladder following “chemical sympathectomy”. Acta Pharmacol Toxicol 44: 377–384
Finney DJ (1964) Statistical method in biological assay. Charles Griffin Ltd, London
Fleming WW (1980) The electrogenic NA+, K+-pump in smooth muscle: physiologic and pharmacologic significance. Ann Rev Pharmacol Toxicol 20:129–149
Fleming WW, McPhilips JJ, Westfall DP (1973) Postjunctional supersensitivity and subsensitivity of excitable tissues of drugs. Ergeb Physiol 68:55–119
Frigo GM, Lecchini S (1970) An improved method for studying the peristaltic reflex in the isolated colon. Br J Pharmacol 39:346–356
Frigo GM, Torsoli A, Lecchini S, Falaschi CF, Crema A (1972) Recent advances in the pharmacology of peristalsis. Arch Int Pharmacodyn Ther 196 [Suppl]:9–24
Gabella G (1979) Innervation of the gastrointestinal tract. Int Rev Cytol 59:129–193
Gabella G (1981) Structure of muscles and nerves in the gastrointestinal tract. In: Johnson LR (ed) Physiology of the gastrointestinal tract. Raven Press, New York, pp 197–241
Gonella J, Lecchini S (1971) Inhibition de l'activité électrique de la couche circulaire du duodénum de Lapin, in vitro, par stimulation des fibres sympathiques périatérielles du mésentère. CR Acad Sci (Paris) 273:214–217
Green RD, Fleming WW, Schmidt JL (1968) Sensitivity changes in the isolated ileum of the guinea-pig after pretreatment with reserpine. J Pharmacol Exp Ther 162:270–276
Harpley FW, Stewart GA, Young PA (1973) Principles of biological assay. In: Delaunois AI (ed) Biostatistics in pharmacology, vol 2. Pergamon Press, Oxford New York, pp 971–1060
Langer SZ (1981) Presynaptic regulation of the release of catecholamines. Pharmacol Rev 32:337–362
Langer SZ Dubocovich ML (1981) Cocaine and amphetamine antagonize the decrease of noradrenergic neutrotransmission elicited by oxymetazoline but potentiate the inhibition by α-metylnorepinephrine in the perfused cat spleen. J Pharmacol Exp Ther 216:162–171
Lecchini S, Del Tacca M, Soldani G, Frigo GM, Crema A (1969) The actions of atropine, tropenziline and N-butyl hyoscine bromide on the isolated distal colon of the guinea-pig: a comparison of their activities and mechanisms of action. J Pharm Pharmacol 21:662–667
Mazzanti L, Del Tacca M, Breschi MC, Frigo GM, Friedman C, Crema A (1972) The time course of functional and morphological changes of the guinea-pig colon after “a frigore” denervation of the periarterial sympathetic nerves. Acta Neuropathol (Berl) 22:190–199
Medgett IC, McCulloch MW, Rand MJ (1978) Partial agonist action of clonidine on prejunctional and postjunctional α-adrenoceptors. Naunyn-Schmiedeberg's Arch Pharmacol 304:215–221
Paton WDM, Vizi ES (1969) The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol 35:10–28
Pluchino S, Trendelenburg U (1968) The influence of denervation and of decentralization on the alpha and beta effects of isoproterenol in the nictitating membrane of the pithed cat. J Pharmacol Exp Ther 163:257–265
Roskowski AP, Koelle GB (1960) Enhancement of inhibitory and excitatory effects of catecholamines. J Pharmacol Exp Ther 128:227–232
Sanger GJ, Bennett A (1982) In vitro techniques for the study of gastrointestinal motility. In: Bertaccini G (ed) Mediators and drugs in gastrointestinal motility. Handbook of experimental pharmacology, vol 59/1. Springer, Berlin Heidelberg New York, pp 205–222
Sax RD, Wastfall TC (1981) Alterations in prejunctional adrenoceptor function in guinea-pig and rat vas deferens after chronic ganglionic blockade. J Pharmacol Exp Ther 219:21–26
Starke K, Wagner J, Schümann H (1972) Adrenergic neuron blockade by clonidine: Comparison with guanethidine and local anaesthetics. Arch Int Pharmacodyn Ther 195:291–308
Stjärne L (1975) Clonidine enhances the secretion of sympathetic neurotransmitter from isolated guinea-pig tissues. Acta Physiol Scand 93:142–144
Trendelenburg U (1972) Factors influencing the concentration of catecholamines at the receptors. In: Blaschko H, Muscholl E (eds) Catecholamines. Handbook of experimental pharmacology, vol 33. Springer, Berlin Heidelberg New York, pp 726–762
Trendelenburg U (1980) Supersensitivity in peripheral organs. In: Pepeu G, Kuhar MJ, Enna SJ (eds) Receptors for neurotransmitters and peptide hormones. Raven Press, New York, pp 99–105
Westfall DP (1981) Supersensitivity of smooth muscle. In: Bulbring E, Brading AF, Jones AW, Tomita T (eds) Smooth muscle: An assessment of current knowledge. Edward Arnold Ltd, London, pp 285–309
Wikberg JES (1978) Differentiation between pre- and postjunctional α-receptors in guinea-pig ileum and rabbit aorta. Acta Physiol Scand 103:225–239
Wikberg JES (1979) The pharmacological classification of adrenergic α1 and α2 receptors and their mechanisms of action. Acta Physiol Scand 468 [Suppl]:1–92
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Frigo, G.M., Lecchini, S., Marcoli, M. et al. Changes in sensitivity to the inhibitory effects of adrenergic agonists on intestinal motor activity after chronic sympathetic denervation. Naunyn-Schmiedeberg's Arch. Pharmacol. 325, 145–152 (1984). https://doi.org/10.1007/BF00506194
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00506194