Summary
The effects of dopamine (DA) were studied on guinea-pig isolated tracheal chains. At a low concentration (10−6M) DA occasionally produced a small contraction; this was followed by a dose-dependent relaxation 3×10−6–3×10−3 M).
On a molar basis, DA was about 40 times less potent than noradrenaline (NA) in relaxing tracheal chains, and about 2700 times less potent than isoprenaline (ISO). The maximum degree of relaxation obtained with each drug was the same.
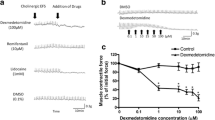
Pretreatment of the guinea-pig with reserpine (5 mg/kg) resulted in a 3-fold shift of the DA curve to the right without concomitantly affecting the ISO doseresponse curve. Reserpine completely abolished the relaxant effects of tyramine, but a small contractile response remained.
Desipramine (DMI), at a concentration of 10−5 M, caused a 4-fold shift of the DA curve to th right. The same concentration of DMI resulted in a shift to the left of the NA dose-response curve by about 8-fold. Benztropine (10−5 M) and haloperidol (10−5 M and 3×10−5 M) did not affect the DA dose-response curve.
The DA-induced relaxation was inhibited by propranolol (10−8–10−6 M) in a dose-dependent manner. The higher concentrations of propranolol (10−7 and 10−6 M) unmasked the contractile effect of DA. In the presence of propranolol, phentolamine (10−5 M) abolished the contractile effect of DA.
It is concluded that DA has both direct and indirect actions on guinea-pig isolated tracheal smooth muscle. The relaxant effects of DA are predominantly due to a direct action on smooth muscle β-adrenoceptors, with a component due to release of NA from adrenergic nerves. The contractile effects, which under normal conditions are masked by the relaxant effect of DA, are mediated by functional α-adrenoceptors. There is no evidence for either specific dopaminergic nerves, uptake mechanisms or receptors in guinea-pig trachealis muscle.
Similar content being viewed by others
References
Akcasu A (1959) The physiologic and pharmacologic characteristics of the tracheal muscle. Arch Int Pharmacodyn 122:201–207
Arunlakshana O, Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14:48–58
Downes H, Loehning RW (1977) Local anesthetic contracture and relaxation of airway smooth muscle. Anesthesiology 47:430–436
Endoh M (1975) Effects of dopamine on sinus rate and ventricular contractile force of the dog heart in vitro and in vivo. Br J Pharmacol 55:475–486
Eyre P, Deline TR (1971) Release of dopamine from bovine lung by specific antigen and by compound 48/80. Br J Pharmacol 42:423–427
Falck B, Nystedt T, Rosengren E, Stenflo J (1964) Dopamine and mast cells in ruminants. Acta Pharmacol Tox 21:51–58
Fleisch JH, Maling HM, Brodie BB (1970) Evidence for existence of alpha-adrenergic receptors in the mammalian trachea. Am J Physiol 218:596–599
Godberg LI (1972) Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev 24:1–29
Goldberg LI, Volkman PH, Kohli JD (1978) A comparison of the vascular dopamine receptor with other dopamine receptors. Ann Rev Pharmacol Tox 18:57–79
Harms HH (1976) Isoproterenol antagonism of cardioselective beta adrenergic receptor blocking agents: A comparative study of human and guinea-pig cardiac and bronchial beta adrenergic receptors. J Pharmacol Exp Ther 199:329–335
Henderson WR, Shelhamer HJ, Reingold DB, Smith LJ, Evans R, III Kaliner M (1979) Alpha-adrenergic hyper-responsiveness in asthma. Analysis of vascular and pupillary responses. New Engl J Med 300:642–647
Horn AS, Coyle JT, Snyder SH (1971) Catecholamine uptake by synaptosomes from rat brain; structure-activity relationships of drugs with differential effects on dopamine and norepinephrine neurons. Mol Pharmacol 7:66–80
Iversen LL (1973) Catecholamine uptake processes. Br Med Bull 29:130–135
Kneussl MP, Richardson JB (1978) Alpha-adrenergic receptors in human and canine tracheal and bronchial smooth muscle. J Appl Physiol 45:307–311
McNay JL, Goldberg LI (1966) Comparison of effects of dopamine, isoproterenol, norepinephrine and bradykinin on canine renal and femoral blood flow. J Pharmacol Exp. Ther 151:23–31
O'Donnell SR, Saar N (1973) Histochemical localization of adrenergic nerves in the guinea-pig trachea. Br J Pharmacol 47:707–710
Tattersfield AE (1979) Airway Pharmacology. Br J Anaesth 51:681–691
Tenner TE (1979) Reserpine induced cardiac supersensitivity. Proc West Pharmacol Soc 22:1–4
Thomson NC, Patel KR (1978) Effect of dopamine on airways conductance in normals and extrinsic asthmatics. Br J Clin Pharmacol 5:421–424
Thorner MO (1975) Dopamine is an important neurotransmitter in the autonomic nervous system. Lancet 2:662–664
Trendelenburg U (1972) Factors influencing the concentration of catecholamines at the receptors. Handb Exp Pharmak 33:726–761
Tsai TH, Langer SZ, Trendelenburg U (1967) Effects of dopamine and α-methyl-dopamine on smooth muscle and on the cardiac pacemaker. J Pharmacol Exp Ther 156:310–324
Wakade AR (1980) A comparison of rates of depletion and recovery of noradrenaline stores of peripheral and central noradrenergic neurones after reserpine administration: importance of neuronal activity. Br J Pharmacol 68:93–98
Westfall DP, Fleming WW (1968) The sensitivity of the guinea-pig pacemaker to norepinephrine and calcium after pretreatment with reserpine. J Pharmacol Exp Ther 164:259–269
Yeh BK, McNay JL, Goldberg LI (1969) Attenuation of dopamine renal and mesenteric vasodilation by haloperidol: evidence for a specific dopamine receptor. J Pharmacol Exp Ther 168:303–309
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koga, Y., Downes, H. & Taylor, S.M. Direct and indirect actions of dopamine on tracheal smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 315, 15–20 (1980). https://doi.org/10.1007/BF00504225
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00504225