Summary
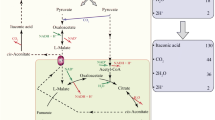
The mechanism of the control of citric acid accumulation by oxygen was investigated by means of pilot plant fermentation using Aspergillus niger. The critical dissolved oxygen tension (DOT) for oxygen uptake of this fungus was about 18–21 and 23–26 mbar for trophophase and idiophase, respectively. Minimal DOT for citric acid production was about 25 mbar. Citric acid production increased steadily between 40–150 mbar. Short time changes in the DOT produced immediate, irreversible changes in the rate of product formation. Adenine nucleotides paralleled growth but showed no evidence for control function in the oxygen effect on citric acid fermentation. A branched respiratory system was identified by experiments using specific inhibitors (antimycin, cyanide, azide, rotenone, amytal and salicylhydroxamic acid). Growth was sensitive towards inhibitors of the standard respiratory chain, but only slightly sensitive towards salicylhydroxamic acid (SHAM). Citric acid synthesis was highly sensitive towards SHAM during trophophase, but sensitive towards antimycine during idiophase. Interruptions in aeration cause an impairment of the SHAM sensitive oxidase during trophophase, and of the antimycin sensitive oxidase during idiophase.
Similar content being viewed by others
References
Bull AT, Bushell ME (1975) In: Smith JE, Berry DR (eds) The filamentous fungi II. Edward Arnold, London, pp 1–31
Carter BLA, Bull AT (1969) Biotechnol Bioeng 11:785–804
Carter BLA, Bull AT (1971) J Gen Microbiol 65:265–273
Clark DS, Leutz CP (1961) Can J Microbiol 7:447–453
Edwards DL, Kwiecinsky F (1973) J Bacteriol 116:610–618
Edwards DL, Unger BW (1978) J Bacteriol 133: 1130–34
Fiechter A, Meyenburg K Von (1968) Biotechnol Bioeng 10: 535–549
Habison A, Kubicek CP, Röhr M (1979) FEMS Microbiol Letts 5:39–42
Kawakita M (1970) J Biochem 68:625–31
Khan AH, Ghose TK (1973) J Ferment Technol 51:734–41
Kristiansen B, Sinclair CG (1979) Biotechnol Bioeng 21:297–315
Kubicek CP, Röhr M (1978) Eur J Appl Micribiol Biotechnol 5:263–271
Kubicek CP, Zehentgruber O, Röhr M (1979) Biotechnol Lett 1:47–52
Lambowitz AM, Slayman CW (1971) J Bacteriol 108:1087–96
Lambowitz AM, Smith EW, Slayman CW (1972) J Biol Chem 247:4859–65
Lundin A, Thorne A (1974) Anal Biochem 66:47–56
Maresca B, Lambowitz AM, Kobayashi GS, Medoff G (1979) J Bacteriol 138:647–49
Phillips DH, Johnson MJ (1961) Biochem Microbiol Technol Eng 3:277–309
Rogers PR, Clarke-Walker GD, Stewart PR (1974) J Bacteriol 119:282–293
Rogers PR, Gleason FH (1974) Mycologia 66:919–925
Rowley BI, Pirt SJ (1972)J Gen Microbiol 72:553–563
Shu P (1953) J Agric Food Chem 1:1119–1126
Tempest DW, Neijssel OM (1979) Biochem Soc Trans 7:82–85
Umbreit WW, Burris RH, Stauffer JF (1964) Manometric techniques, 4th edn. Minneapolis: Burgess
Watson K (1975) In: Smith JE, Berry DR (eds) The filamentous fungi II. Edward Arnold, London, pp 92–120
Watson, K, Smith JE (1967a) Biochem J 104:332–339
Watson K, Smith JE (1967b) J Biochem 61:527–530
Williamson JR, Corckey BE (1973). In: Lowenstein JM (ed) Methods in enzymology, Vol. 13. Academic Press, New York, pp 434–513
Zetelaki KZ (1970) Biotechnol Bioeng 12:379–397
Author information
Authors and Affiliations
Additional information
Dedicated to emeritus Professor Dr. Richard Brunner on the occasion of his 80th birthday
Rights and permissions
About this article
Cite this article
Kubicek, C.P., Zehentgruber, O., El-Kalak, H. et al. Regulation of citric acid production by oxygen: Effect of dissolved oxygen tension on adenylate levels and respiration in Aspergillus niger . European J. Appl. Microbiol. Biotechnol. 9, 101–115 (1980). https://doi.org/10.1007/BF00503505
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00503505