Summary
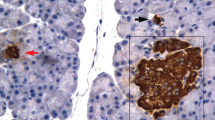
Secretory granules of pancreatic B-cells contain high concentrations of zinc and calcium. The effect of gradual degranulation (induced by tolbutamide over a period of 3 days) and the subsequent regranulation (over a period of 4 days) on the histochemically detectable zinc (Zn) and calcium (Ca) content of B-cells was investigated. Zn was stained by dithizone, Ca by glyoxal-bis-(2-hydroxyanil), (GBHA), and B-granules by aldehyde fuchsin (AF). The staining intensities were determined cytophotometrically. A decrease of the granulation by 50% causes a comparable decrease of the Zn content. Almost complete degranulation, however, hardly further diminished the Zn content. Regranulation restores the Zn content parallel to the granulation. The presence of 40% of the initial Zn content in degranulated B-cells suggests the existence of a non-granular Zn fraction. The Zn content of B-cells may be partly involved in the storage of insulin as a Zn-insulin complex in the secretory vesicles. A-cells, however, contain even more (+30%) Zn than B-cells. Degranulation of B-cells is accompanied by a moderate decrease of the zinc content of the A-cells. The function of Zn in A-cells is completely unknown. Degranulation of B-cells causes the GBHA-Ca content to decrease to a very low level parallel to the AF-positive granulation. During regranulation the GBHA-Ca content restores parallel to the granulation and reaches after complete regranulation a slightly higher level than in untreated control rats. Almost complete disappearance of CBHA-Ca in the B-cells is accompanied by a decrease of the total islet calcium content of 33%. The results indicate that GBHA stains a Ca fraction which is mainly localized to the secretory granules. The stainability of granular Ca by GBHA is probably based on: a) the high Ca concentration in the granules, b) the presence of ionized Ca in the granules, due to the low intragranular pH, and c) on the properties of GBHA, which stains, under conditions used, only ionized (possibly also readily ionizable) Ca.
Similar content being viewed by others
References
Bänder A, Schesmer G (1970) Histochemical evidence for a sulfonylurea effect on the zinc content of the pancreatic islets. Wenner-Gren Center International Symposium Series vol. 16. In: Falkmer S, Hellman B, Täljedal I-B (eds) The structure and metabolism of the pancreatic islets. Pergamon Press, Oxford, pp 190–202
Bommer G, Joost HG, Klöppel G (1978) Subcellular B-cell calcium and insulin secretion in vitro. Comparative ultracytochemical studies after glucose stimulation and cyproheptadine inhibition. Virchows Arch Pathol Anat Histol 379:203–217
Bosboom RS, Zweens J, Bouman PR (1973) Effects of feeding and fasting on the insulin secretory response to glucose and sulfonylureas in intact rats and isolated perfused rat pancreas. Diabetologia 9:243–250
Chalkley HW (1943) Method for quantitative morphologic analysis of tissues. J Natl Cancer Inst 4:47–53
Emdin SO, Dodson GG, Cutfield JM, Cutfield SM (1980) Role of zinc in insulin biosynthesis. Diabetologia 19:174–182
Freie HMP, Pasma A, Bouman PR (1975) Quantitative analysis of pancreatic islet development and insulin storage in the foetal and newborn rat. Acta Endocrinol 80:657–666
Fujimoto WY, Ensinck JW (1981) Regulation of A- and B-cell function by insulin and glucagon. Horm Metab Res 13:547–550
Grant PT, Coombs TL, Frank BH (1972) Differences in the nature of the interaction of insulin and proinsulin with zinc. Biochem J 126:433–440
Hartroft WS, Wrenshall GA (1955) Correlation of beta-cell granulation with extractable insulin of the pancreas. Diabetes 4:1–7
Hellman B, Lenzen S, Sehlin J, Täljedal I-B (1977) Effects of various modifiers of insulin release on the lanthanum-nondisplaceable 45Ca2+ uptake by isolated pancreatic islets. Diabetologia 13:49–53
Hellman B, Andersson T, Berggren P-O, Flatt P, Gylfe E, Kohnert K-D (1979) The role of calcium in insulin secretion. In: Dumont J, Nunez J (eds) Hormones and cell regulation. Vol 3. Elsevier North-Holland, Biomedical Press, Amsterdam, pp 69–95
Henquin J-C (1980) Tolbutamide stimulation and inhibition of insulin release. Studies of the underlying ionic mechanisms in isolated rat islets. Diabetologia 18:151–160
Herman L, Sato T, Hales CN (1973) The electron microscopic localization of cations to pancreatic islets of Langerhans and their possible role in insulin secretion. J Ultrastruct Res 42:298–311
Howell SL, Montague W, Tyhurst M (1975) Calcium distribution in islets of Langerhans: A study of calcium concentrations and of calcium accumulation in B cell organelles. J Cell Sci 19:395–409
Howell SL, Tyhurst M, Duvefelt H, Andersson A, Hellerström C (1978) Role of zinc and calcium in the formation and storage of insulin in the pancreatic β-cell. Cell Tissue Res 188:107–118
Hutton JC (1982) The internal pH and membrane potential of the insulin-secretory granule. Biochem J 204:171–178
Hutton JC, Penn EJ, Peshavaria M (1983) Low-molecular-weight constituents of isolated insulin-secretory granules. Biochem J 210:297–305
Klöppel G, Schäfer H-J (1976) Effects of sulfonylureas on histochemical and ultracytochemical calcium distribution in B-cells of mice. Diabetologia 12:227–235
Klöppel G, Bommer G, Ruttmann E, Schäfer H-J (1978) Qualitative and semiquantitative calcium cytochemistry in B-cells of mice treated with cyproheptadine and mannoheptulose. Acta Endocr 87:786–798
Klöppel G, Bommer G, Lenzen S (1980) Calcium ultracytochemistry in pancreatic B-cells. Horm Metab Res (Suppl) 10:138–144
Kvistberg D, Lester G, Lazarow A (1966) Staining of insulin with aldehyde fuchsin. J Histochem Cytochem 14:609–611
Lazarus SS, Volk BW (1970) Ultrastructural aspects of the function of rabbit B-cells. In: Falkmer S, Hellman B, Täljedal I-B (eds) The structure and metabolism of the pancreatic islet. Pergamon Press, Oxford, pp 159–170
Malaisse WJ, Mahy M, Brisson GR, Malaisse-Lagae F (1972) The stimulus-secretion coupling of glucose-induced insulin secretion. VII. Combined effects of glucose and sulfonylureas. Eur J Clin Invest 2:85–90
Malaisse WJ, Herchuelz A, Devis G, Somers G, Boschero AC, Hutton JC, Kawazu S, Sener A (1978) Regulation of calcium fluxes and their regulatory roles in pancreatic islets. Ann NY Acad Sci 307:562–583
Pihl E (1968) An ultrastructural study of the distribution of heavy metals in the pancreatic islets as revealed by the sulfide silver method. Acta Pathol Microbiol Scand 74:145–160
Ravazzola M, Malaisse-Lagae F, Amherdt M, Perrelet A, Malaisse WJ, Orci L (1976) Patterns of calcium localization in pancreatic endocrine cells. J Cell Sci 27:107–117
Rosenbloom AL, Rennert OM (1970) Specificity and sensitivity of insulin staining by aldehyde fuchsin, pseudocyanin and toluidine blue. Stain Technol 45:25–27
Schäfer H-J, Klöppel G (1974) Demonstration of calcium in pancreatic islets. Light microscope observations in activated and inactivated B-cells of mice. Virchows Arch Pathol Anat Histol 362:1–12
Scott HR, Clayton BP (1953) A comparison of the staining affinities of aldehyde-fuchsin and the Schiff reagent. J Histochem Cytochem 1:336–352
Wolters GHJ, Konijnendijk W, Bouman PR (1977) Effects of fasting on insulin secretion, islet glucose metabolism and the cyclic adenosine 3′, 5′-monophosphate content of rat pancreatic islets in vitro. Diabetes 26:530–537
Wolters GHJ, Pasma A, Konijnendijk W, Boom G (1979a) Calcium, zinc and other elements in islet and exocrine tissue of the rat pancreas as measured by histochemical methods and electron-probe micro-analysis. Effects of fasting and tolbutamide. Histochemistry 62:1–17
Wolters GHJ, Pasma A, Konijnendijk W, Bouman PR (1979b) Evaluation of the glyoxal-bis-(2-hydroxyanil)-method for staining of calcium in model gelatin films and pancreatic islets. Histochemistry 62:137–151
Wolters GHJ, Pasma A, Konijnendijk W, Bouman PR (1980) Effects of calcium manipulation and glucose stimulation on a histochemically detectable mobile calcium fraction in isolated rat pancreatic islets. Histochemistry 66:125–135
Wolters GHJ, Pasma A, Konijnendijk W, Bosman F (1981) Functional subdivision of the pancreatic islet population of the rat. Neth J Med 24:163–164
Wolters GHJ, Vonk M, Konijnendijk W (1982a) Histochemical calcium content of pancreatic islets and impaired insulin secretion. Neth J Med 25:286 (abstract)
Wolters GHJ, Wiegman JB, Konijnendijk W (1982b) The effect of glucose stimulation on 45Calcium uptake of rat pancreatic islets and their total calcium content as measured by a fluorometric micro-method. Diabetologia 22:122–127
Wolters GHJ, Pasma A, Konijnendijk W (1983) Decreased response of mobile calcium in pancreatic islets of fasted rats to glucose stimulation and calcium manipulation. Diabetes 32:235–240
Yokoh S, Aoji O, Matsuno Z, Yoshida H (1969) Electron microscopic histochemistry of heavy metals in islets of Langerhans of rabbits. Diabetologia 5:137–142
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wolters, G.H.J., Pasma, A., Wiegman, J.B. et al. Changes in histochemically detectable calcium and zinc during tolbutamide-induced degranulation and subsequent regranulation of rat pancreatic islets. Histochemistry 78, 325–338 (1983). https://doi.org/10.1007/BF00496620
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00496620