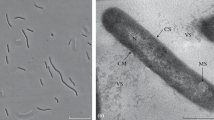
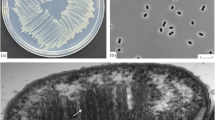
Abstract
A new mesophilic, monotrichously flagellated methane-producing coccus of ≦1μm in diameter was isolated from an anaerobic sour whey digester, originally inoculated with sewage sludge. Growth and methane production were observed with H2/CO2, formate and — less effectively — with 2-propanol/CO2. The isolate grew at temperatures between 15° C and 45° C with the optimum at around 37° C. Acetate, yeast extract and tungstate were required in the medium. Clarified rumen fluid stimulated growth.
The DNA of the new methanogen has a G+C content of 48.5 mol%. Comparative 16 S rRNA oligonucleotide cataloguing allows to define the new isolate as a member of a new genus of the order Methanomicrobiales. Further evidence for this is provided by the antigenic crossreactivity with anti-S probes and by metabolic features.
Because of its small size the new methanogen is named Methanocorpusculum parvum.
Similar content being viewed by others
References
Aranki A, Freter R (1972) Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr 25:1329–1334
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: Reevaluation of a unique biological group. Microbiol Rev 43:260–296
Birnboim C, Doly J (1979) Rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Blaut M, Gottschalk G (1984) Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem 141:217–222
Blaut M, Müller V, Fiebig K, Gottschalk G (1985) Sodium ions and an energized membrane required by Methanosarcina barkeri for the oxidation of methanol to the level of formaldehyde. J Bacteriol 164:95–101
Bray GA (1960) A simple efficient liquid scintillator for counting aqueous solutions in a liquid scintillation counter. Anal Biochem 1:279–285
Burke KA, Calder K, Lascelles J (1980) Effects of molybdenum and tungstate on induction of nitrate reductase and formate dehydrogenase in wild type and mutant Paracoccus denitrificans. Arch Microbiol 126:155–159
Conway de Macario E, Macario AJL, Wolin MJ (1982a) Antibody analysis of relationships among methanogenic bacteria. J Bacteriol 149:316–319
Conway de Macario E, Macario AJL, Wolin MJ (1982b) Specific antisera and immunological procedures for characterization of methanogenic bacteria. J Bacteriol 149:320–328
Conway de Macario E, Macario AJL, Jovell RJ (1983) Quantitative slide micro-immunoenzymatic assay (micro SIA) for antibodies to particulate and non particulate antigens. J Immunol Meth 59:93–47
Corder RE, Hook LA, Larkin JM, Frea JI (1983) Isolation and characterization of two new methane producing cocci: Methanogenium olentangyi, sp. nov., and Methanococcus deltae, sp. nov. Arch Microbiol 134:28–32
Diekert GD, Weber B, Thauer RK (1980) Nickel dependence of factor F430 content in Methanobacterium thermoautotrophicum. Arch Microbiol 127:273–278
Fox GE, Pechman KR, Woese CR (1977) Comparative cataloguing of 16 S ribosomal ribonucleic acid: molecular approach to procaryotic systematics. Int J Syst Bacteriol 27:44–57
Graf EG, Thauer RK (1981) Hydrogenase from Methanobacterium thermoautotrophicum, a nickel-containing enzyme. FEBS Lett 136:165–169
Hammel KE, Cornell KL, Diekert GB, Thauer RK (1984) Evidence for a nickel-containing carbon monoxide dehydrogenase in Methanobrevibacter arboriphilicus. J Bacteriol 157:975–978
Huser BA, Wuhrmann K, Zehnder AJB (1982) Methanothrix soehngenii gen. nov., spec. nov., a new acetotrophic, non hydrogen oxidizing methane bacterium. Arch Microbiol 132:1–9
Jones JB, Stadtman TC (1977) Methanococcus vannielii: Culture and effects of selenium and tungsten on growth. J Bacteriol 130:1404–1406
Jones JB, Stadtman TC (1981) Selenium-dependent and selenium-independent formate dehydrogenase of Methanococcus vannielii. J Biol Chem 256:656–663
Keltjens JT, van der Drift (1986) Electron transfer reactions in methanogens. FEMS Microbiol Rev 39:259–303
König H, Stetter KO (1982) Isolation and characterization of Methanolobus tindarius sp. nov., a coccoid methanogen growing only on methanol and methylamines. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt I Orig C 3:478–490
Kühn W, Gottschalk G (1983) Characterization of the cytochromes occurring in Methanosarcina species. Eur J Biochem 135:89–94
Kühn W, Fiebig K, Walther R, Gottschalk G (1979) Presence of a cytochrome b 559 in Methanosarcina barkeri. FEBS Lett 105:271–274
Kühn W, Fiebig K, Hippe H, Mah RA, Huser BA, Gottschalk G (1983) Distribution of cytochromes in methanogenic bacteria. FEMS Microbiol Lett 20:407–410
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
McGill TJ, Jurka J, Sobieski JM, Pickett MH, Woese CR, Fox GE (1986) Characteristic archaebacterial 16 S rRNA oligonucleotides. Syst Appl Microbiol 7:194–197
Miller TL, Wolin MJ (1985) Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol 141:116–122
Paynter MJB, Hungate RE (1968) Characterization of Methanobacterium mobilis, sp. nov., isolated from bovine rumen. J Bacteriol 95:1943–1951
Rivard CJ, Henson JM, Thomas MV, Smith PH (1983) Isolation and characterization of Methanomicrobium paynteri sp. nov., a mesophilic methanogen isolated from marine sediments. Appl Environ Microbiol 46:484–490
Romesser JA, Wolfe RS, Mayer F, Spiess E, Walther-Mauruschat A (1979) Methanogenium, a genus of marine methanogenic bacteria and characterization of Methanogenium cariaci spec. nov. and Methanogenium marisnigri spec. nov. Arch Microbiol 121:147–153
Scherer P, Sahm H (1981) Effect of trace minerals and vitamins on the growth of Methanosarcina barkeri. Acta Biotechnol 1:57–65
Schönheit P, Moll J, Thauer RK (1979) Nickel, cobalt and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol 123:105–107
Scott RH, Sperl GT, DeMoss JA (1979) In vitro incorporation of molybdate into demolybdo proteins in Escherichia coli. J Bacteriol 137:719–726
Sowers KR, Ferry FG (1985) Trace metal and vitamin requirements of Methanococcoides methylutens grown with trimethylamine. Arch Microbiol 142:148–151
Sowers KR, Johnson JL, Ferry JG (1984) Phylogenetic relationship among the methylotrophic methane-producing bacteria and emendation of the family Methanosarcinaceae. Int J Syst Bacteriol 34:444–450
Stackebrandt E, Woese CR (1981) The evolution of prokaryotes. In: Carlile MJ, Collins JF, Moseley BEB (eds) Molecular and cellular aspects of microbial evolution (Society for General Microbiology Symposium 32). Cambridge, Cambridge University Press, pp 1–31
Stackebrandt E, Seewaldt E, Ludwig W, Schleifer KH, Huser BA (1982) The phylogenetic position of Methanothrix soehngenii elucidated by a modified technique of sequencing oligonucleotides from 16 S rRNA. Zentralbl Bakt Hyg, I. Abt Orig C 3:90–100
Stackebrandt E, Ludwig W, Fox GE (1985) 16 S ribosomal RNA oligonucleotide cataloguing. In: Gottschalk G (ed) Methods in microbiology. Academic Press, London, pp 75–107
Van Bruggen JJA, Zwart KB, Hermans JGF, Van Hove EM, Stumm CK, Vogels GD (1986) Isolation and characterization of Methanoplanus endosymbiosum sp. nov., an endosymbiont of the marine sapropelic ciliate Metopus contortus Quennerstedt. Arch Microbiol 144:367–374
Wei-Mei Ching, Wittwer AJ, Lin Tsai, Stadtman TC (1984) Distribution of two selenonucleotides among the selenium containing tRNAs from Methanococcus vannielii. Proc Natl Acad Sci USA 81:57–60
Whitman WB, Wolfe RS (1980) Presence of nickel in factor F430 from Methanobacterium bryantii. Biochem Biophys Res Common 92:1196–1201
Widdel F (1986) Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol 51:1056–1062
Wildenauer FX, Winter J (1985) Anaerobic digestion of high-strength acidic whey in a pH controlled up-flow anaerobic fixed film loop reactor. Appl Microbiol Biotechnol 22:367–372
Wildgruber G, Thomm M, König H, Ober K, Ricchiuto T, Stetter KO (1982) Methanoplanus limicola, a plate-shaped methanogen representing a novel family, the Methanoplanaceae. Arch Microbiol 132:31–36
Winter J (1980) Glucose fermentation to methane and CO by defined mixed cultures. Zentralbl Bakt Hyg I. Abt C 1:201–214
Winter J, Lerp C, Zabel HP, Wildenauer FX, König H, Schindler F (1984) Methanobacterium wolfei, sp. nov., a new tungstenrequiring, thermophilic, autotrophic methanogen. System Appl Microbiol 5:457–466
Yamazaki S (1982) A selenium-containing hydrogenase from Methanococcus vannielii. J Biol Chem 257:7926–7929
Zabel HP, König H, Winter J (1984) Isolation and characterization of a new coccoid methanogen, Methanogenium tatii spec. nov. from a solfataric field on Mount Tatio. Arch Microbiol 137:308–315
Zabel HP, König H, Winter J (1985) Emended description of Methanogenium thermophilicum, Rivard and Smith, and assignment of new isolates to this species. System Appl Microbiol 6:72–78
Zellner G, Winter J (1987) Analysis of a highly efficient methanogenic consortium producing biogas from whey. Syst Appl Microbiol (in press)
Author information
Authors and Affiliations
Additional information
This work was supported by a grant of the Deutsche Forschungsgemeinschaft DFG to J. W. and E. S. Immunologic studies were supported in part by grants No. DE-FGO2-84 R 13197 from the U.S. Department of Energy, and No. 261.81/82 from the North Atlantic Treaty Organization (NATO)
Rights and permissions
About this article
Cite this article
Zellner, G., Alten, C., Stackebrandt, E. et al. Isolation and characterization of Methanocorpusculum parvum, gen. nov., spec. nov., a new tungsten requiring, coccoid methanogen. Arch. Microbiol. 147, 13–20 (1987). https://doi.org/10.1007/BF00492898
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00492898