Summary
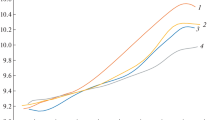
During a 5-month period the polymerization rate of monomeric glutaraldehyde was determined at different temperatures and pHs. To elucidate the role of temperature, the authors used a 20.8% aqueous solution of glutaraldehyde at the following temperatures: −14°, 4°, 20°, 25°, 30°, 40°, 50°, and 60° C. With increasing temperature the rate of polymerization rose exponentially. To elucidate the role of pH the authors used 4% glutaraldehyde solutions in Teorell and Stenhagen's universal buffer of the following pHs: 3.0, 5.0, 6.5, 7.5, 8.5, 10.0, and 12.0 and in phosphate and cacodylate buffers pH 7.4. On both sides of the neutral point the polymerization rate showed a marked rise, so that the pH dependence could be depicted as an exponential curve. Moreover, a marked difference in the polymerization rate was found for the various buffers at the same pH. It was not possible to demonstrate a significant decrease of the osmolar concentration within the experimental range gradually as the polymerization increased.
Similar content being viewed by others
References
Anderson, P. J.: Purification and quantitation of glutaraldehyde and its effects on several enzyme activities in skeletal muscle. J. Histochem. Cytochem. 15, 652–661 (1967a)
Anderson, P. J.: Standardization of glutaraldehyde and evaluation of its enzyme inhibitory effects in skeletal muscle. J. Histochem. Cytochem. 15, 789–790 (1967b)
Fahimi, H. D., Drochmans, P.: Essais de standardisation de la fixation au glutaraldehyde. I. Purification et determination de la concentration du glutaraldehyde. J. Micr. 4, 725–736 (1965)
Fahimi, H. D., Drochmans, P.: Purification of glutaraldehyde. Its significance for preservation of acid phosphatase activity. J. Histochem. Cytochem. 16, 199–204 (1968)
Frigerio, N. A., Shaw, M. J.: A simple method for determination of glutaraldehyde. J. Histochem. Cytochem. 17, 176–181 (1968)
Gillett, R., Gull, K.: Glutaraldehyde—its purity and stability. Histochem. 30, 162–167 (1972)
Hardy, P. M., Nicholls, A. C., Rydon, H. N.: The nature of glutaraldehyde in aqueous solution. J. chem. Soc. (D), 565–566 (1969)
Hopwood, D.: The behaviour of various glutaraldehydes on Sephadex G-10 and some implications for fixation. Histochem. 11, 289–295 (1967)
Janigan, D. T.: Tissue enzyme fixation studies. I. True effects of aldehyde fixation on β-glucuronidase, β-galactosidase and β-glucosidase in tissue blocks. Lab. Invest. 13, 1038–1050 (1964)
Janigan, D. T.: The effect of aldehyde fixation on acid phosphatase activity in tissue blocks. J. Histochem. Cytochem. 13, 475–483 (1965)
Pease, D. C.: Histological techniques for electron microscopy, p. 58. New York: Academic Press 1964
Plumel, M.: Tampon au cacodylate de sodium. Bull. Soc. Chim. biol. (Paris) 30, 129–130 (1948)
Richards, F. M., Knowles, J. R.: Glutaraldehyde as a protein cross-linking reagent. J. molec. Biol. 37, 231–233 (1968)
Robertson, E. A., Schultz, R. L.: The impurities in commercial glutaraldehyde and their effect on the fixation of brain. J. Ultrastruct. Res. 30, 275–287 (1970)
Sabatini, D. D., Bensch, K., Barrnett, R. J.: Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 17, 19–58 (1963)
Sabatini, D. D., Miller, F., Barrnett, R. J.: Aldehyde fixation for morphological and enzyme histochemical studies with the electron microscope. J. Histochem. Cytochem. 12, 57–71 (1964)
Smith, R. E., Farquhar, M. G.: Lysosome function in the regulation of the secretory process in cells of the anterior pituitary gland. J. Cell Biol. 31, 319–347 (1966)
Sørensen, S. P. L.: Ergänzung zu der Abhandlung: Enzymstudien II: Über die Messung und die Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen. Biochem. Z. 22, 352–356 (1909)
Teorell, T., Stenhagen, E.: Ein Universalpuffer für den pH-Bereich, 2,0 bis 12,0. Biochem. Z. 299, 416–419 (1938)
Webster, H. de F., Ames, A. III.: Glutaraldehyde fixation of central nervous tissue: an electron microscopic evaluation in the isolated rabbit retina. Tissue and Cell 1, 53–62 (1969)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rasmussen, K.E., Albrechtsen, J. Glutaraldehyde. The influence of pH, temperature, and buffering on the polymerization rate. Histochemistry 38, 19–26 (1974). https://doi.org/10.1007/BF00490216
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00490216