Summary
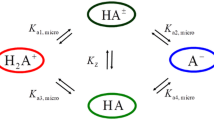
This review summarizes the historical development of approximation formulae proposed for acid-base equilibria in aqueous solutions and introduces our method of calculation concerning the ranges of application of the formulae. The results of calculation are shown as diagrams which will give clear information on the ranges.
Similar content being viewed by others
References
Ostwald W (1894) Die wissenschaftlichen Grundlagen der analytischen Chemie. Verlag von Wilhelm Engelmann, Leipzig
Laitinen HA (1960) Chemical analysis. McGraw-Hill, New York
Freiser H, Fernando Q (1963) Ionic equilibria in analytical chemistry. John Wiley and Sons, New York
Butler JN (1964) Solubility and pH calculations. Addison-Wesley, Reading
Gunther WB (1968) Quantitative chemistry. Addison-Wesley, Reading
Dick JG (1973) Analytical chemistry. McGraw-Hill, New York
Brewer S (1980) Solving problems in analytical chemistry. John Wiley and Sons, New York
Ostwald W (1888) Z Phys Chem 2:270–283
Bredig G (1894) Z Phys Chem 13:289–326
Schoorl N (1921) Rec Trav Chim Pays-Bas 40:616–620
Flood H (1940) Z Elektrochem 46:669–675
Park B (1953) J Chem Educ 30:257–260
House JE, Reiter RC (1968) J Chem Educ 45:679
Charlot G (1947) Anal Chim Acta 1:59–68
DeFord DD (1950) J Chem Educ 27:554–556
DeFord DD (1951) Anal Chim Acta 5:345
DeFord DD (1951) Anal Chim Acta 5:352
Drenan JW (1955) J Chem Educ 32:36
Nightingale ER Jr (1957) J Chem Educ 34:277–280
Claeys A (1962) Anal Chim Acta 27:193
Claeys A (1963) Bull Soc Chim Belg 72:102–122
Claeys A (1963) Bull Soc Chim Belg 72:500–507
Claeys A (1963) Bull Soc Chim Belg 73:23–28
Bruckenstein S, Kolthoff IM (1959) Treatise on analytical chemistry, part I, vol I. Interscience Encyclopedia, New York
Machado AASC (1971) Rev Port Quim 13:19–24
Bliefert C (1978) pH-Wert-Berechnungen. Verlag Chemie, Weinheim, p 73
Doeller S (1980) Anal Chim Acta 115:261–268
Kolthoff IM, Furman NH (1926) Indicators, their uses in quantitative analysis and in the colorimetric determination of hydrogenion concentration. John Wiley and Son, New York, p 11
Kolthoff IM, Rosenblum C (1937) Acid-base indicators. The Macmillan Co., New York, p 19
Arbatsky IW (1938) Fresenius Z Anal Chem 115:117–126
Bjerrum N (1914) Die Theorie der alkalimetrischen und acidimetrischen Titrierungen. Stuttgart
Micaelis L (1922) Die Wasserstoff-Ionen-Konzentration, Teil I. Die theoretischen Grundlagen. Berlin
Noyes AA (1893) Z Phys Chem 11:495–500
Eeckhout J (1952) Anal Chim Acta 7:203–225
Nakayama FS (1970) J Chem Educ 47:67–68
Freiser H (1970) J Chem Educ 47:844–845
Tabbutt FD (1970) J Chem Educ 47:845
Henderson LJ (1908) J Am Chem Soc 30:954–960
Hasselbalch KA (1916) Biochem Z 78:112–144
Van Slyke DD (1922) J Biol Chem 52:525–570
Machado AASC (1971) Rev Port Quim 13:236–241
Claeys AE (1985) Anal Chim Acta 178:277–288
Narasaki H (1979) Talanta 26:605–607
Narasaki H (1980) Talanta 27:187–191
Narasaki H (1980) Talanta 27:193–199
Narasaki H (1980) Talanta 27:409–415
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Narasaki, H. The range of application of the approximation formulae in acid-base equilibria. Z. Anal. Chem. 328, 633–638 (1987). https://doi.org/10.1007/BF00488660
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00488660