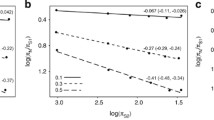
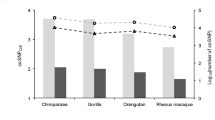
Abstract
During a multipurpose survey we examined electrophoretic mobilities of major (A, i.e., α2β2) and minor (A2, i.e., α2δ2) adult hemoglobins from populations of nine primate genera representing a total of 440 New World monkeys and apes. Sequences of hemoglobin chains were inferred from differences in amino acid composition between homologous tryptic peptides supplemented by detailed placement of more than 270 residues. Beta sequences were thus analyzed in five genera (Aotus, Ateles, Hylobates, Saimiri, and Saguinus) and δ sequences in seven (foregoing plus Gorilla and Pan). In most genera, sequences from several individuals, often from several species, were delineated. Fifteen kinds of intraspecies mutants were detected; 10 of these were precisely characterized. Five of the 15 mutants form electrophoretically detected genetic polymorphisms of δ; none such occur in β. Six electrophoretically detected mutants, four in α and two in δ, are uncommon. One of these represents the complete absence of minor component. Three kinds of variants, two in δ and one in β, are electrophoretically neutral and chance findings during sequence analysis of the equivalent of 38 allele products. Two of the neutral variants are not especially common; one may have polymorphic frequency. Several general conclusions stem from these and supplementary findings. First, comparisons of sequences suggest that δ and β genes in all primates either arose from a single event in a common ancestor or from two approximately coincident events. Either assumption allows reconstruction of a reasonably accurate archetype sequence that is effectively common to all descendants. Second, there is a pancellular quantitative disproportion between major and minor hemoglobins ranging from 16:1 to 220:1 in species studied. Delta is consequently presumed to be functionally and adaptively less vital than β. When these premises are adopted, δ is expected to be relatively invisible to natural selection, and, where darwinism is the principal arbiter of evolution and polymorphism, δ is expected to show fewer fixed changes and fewer genetic polymorphisms than β. The opposite is observed. Delta exhibits as many or more changes from archetype than β. This finding and the comparative abundance of δ polymorphism are attributed to nonadaptive factors which are thus considered the source of much evolutionary change. Third, particular sequence positions in various species are the site of recurrent mutations in both β and δ. One such area is occupied by the majority of genetic polymorphisms found in man and other primates. The overall distribution of mutations arising in evolution is remarkably nonrandom in β, δ, and a pool of both. These results are quite unlike most other observations in higher organisms. The sources of such nonrandomness are either selection and/or differential mutability. We rely on our prior assumption of relative selective invisibility for δ and, in part, ascribe the nonrandom distribution of changes to microzones of enhanced mutability. Fourth, the six uncommon electrophoretically detected mutants provide an estimate of heterozygosity (1/73) at hemoglobin loci that is tenfold greater than observed in man. Fifth, the unprecedented chance detection of three kinds of electrophoretically neutral intraspecies mutants among the equivalent of 38 characterized allele products suggests that neutral changes are as common as electrostatically active ones and at least tenfold more common than expected in extrapolation from human variant surveys. Sixth, β analyses from three kinds of gibbon (Hylobates) hemoglobin suggest that one of these is a potentially unchanged relict of the ancient archetype and, further, indicate a degree of homozygous diversity within a species that nearly equals the difference between gibbon and man.
Similar content being viewed by others
References
Allison, A. C. (1964). Polymorphism and natural selection in human populations. Cold Spring Harbor Symp. Quant. Biol. 29 137.
Auerbach, C. (1967). Changes in the concept of mutation and the aims of mutation research. In Brink, R. A. (ed.), Heritage from Mendel, University of Wisconsin Press, Madison, p. 67.
Benson, J. V., Jr., and Patterson, J. A. (1965). Accelerated automatic chromatographic analysis of amino acids on a spherical resin. Anal. Chem. 37 1108.
Benzer, S. (1961). On the topography of the genetic fine structure. Proc. Natl. Acad. Sci. 47 403.
Boyer, S. H., Fainer, D. C., and Naughton, M. A. (1963a). Myoglobin: Inherited structural variation in man. Science 140 1228.
Boyer, S. H., Rucknagel, D.L., Weatherall, D.J., and Watson-Williams, E.J. (1963b). Further evidence for linkage between the β and δ loci governing human hemoglobin and the population dynamics of linked genes. Am. J. Human Genet. 15 438.
Boyer, S. H., Hathaway, P., and Garrick, M. D. (1964). Modulation of protein synthesis in man: An in vitro study of hemoglobin synthesis by heterozygotes. Cold Spring Harbor Symp. Quant. Biol. 29 333.
Boyer, S. H., Hathaway, P., Pascasio, F., Bordley, J., Orton, C., and Naughton, M. A. (1966). Differences in the amino acid sequences of tryptic peptides from three sheep hemoglobin β chains. J. Biol. Chem. 242 2211.
Boyer, S. H., Crosby, E. F., and Noyes, A. N. (1968). Hemoglobin switching in non-anemic sheep. I. Mediation by plasma from anemic animals. Johns Hopkins Med. J. 123 85.
Boyer, S. H., Crosby, E. F., Fuller, G. F., Noyes, A. N., and Adams, J. G. (1969a). The structure and biosynthesis of hemoglobin A and A2 in the New World primate Ateles paniscus: A preliminary account. Ann. N.Y. Acad. Sci. 165 360.
Boyer, S. H., Crosby, E. F., Thurmon, T. F., Noyes, A. N., Fuller, G. F., Leslie, S. E., Shepard, M. K., and Herndon, C. N. (1969b). Hemoglobins A and A2 in New World primates: Comparative variation and its evolutionary implications. Science 166 1428.
Boyer, S. H., Noyes, A. N., Vrablik, G. R., Donaldson, L. J., and Schaefer, E. W., Jr. (1971). Silent hemoglobin alpha genes in apes: Potential source of thalassemia. Science 171 182.
Carpenter, C. R. (1935). Behavior of red spider monkeys in Panama. J. Mammol. 16 171.
Clarke, B. (1970). Darwinian evolution of proteins. Science 168 1009.
Clegg, J. B., Naughton, M. A., and Weatherall, D. J. (1966). Abnormal human haemoglobins. Separation and characterization of the α and β chains by chromatography, and the determination of two new variants, Hb Chesapeake and Hb J (Bangkok). J. Mol. Biol. 19 91.
Dayhoff, M. O. (1969). Atlas of Protein Sequence and Structure, National Biochemical Research Foundation, Silver Spring, Md.
Doolittle, R. F., Chen, R., Glasgow, C., Mross, G. A., and Weinstein, M. (1970). The molecular constancy of fibrinopeptides A and B from 124 individual humans. Humangenetik 10 115.
Dozy, A. M., Kleihauer, E. F., and Huisman, T. H. J. (1968). Studies on the heterogeneity of hemoglobin. XIII. Chromatography of various human and animal hemoglobin types on DEAE-Sephadex. J. Chromatog. 32 723.
Fitch, W. M., and Margoliash, E. (1967). A method for estimating the number of invariant amino acid coding positions in a gene using cytochrome c as a model case. Biochem. Genet. 1 65.
Gerald, P. S., and Efron, M. L. (1961). Chemical studies of several varieties of Hb-M. Proc. Natl. Acad. Sci. 47 1758.
Gray, W. R., and Smith, J. F. (1970). Rapid sequence analysis of small peptides. Anal. Biochem. 33 36.
Gross, E. (1967). The cyanogen bromide reaction. In Hirs, C. H. W. (ed.), Methods in Enzymology, Vol. XI: Enzyme Structure, Academic Press, New York, p. 238.
Harris, H. (1970). The Principles of Human Biochemical Genetics, North-Holland Publishing Co., Amsterdam, p. 214.
Hoffman, H. A., Gottlieb, A. J., and Wisecup, W. G. (1967). Hemoglobin polymorphism in chimpanzees and gibbons. Science 156 944.
Ingram, V. M. (1963). The Hemoglobins in Genetics and Evolution, Columbia University Press, New York, p. 79.
Jones, R. T. (1964). Structural studies of aminoethylated hemoglobins by automatic peptide chromatography. Cold Spring Harbor Symp. Quant. Biol. 29 297.
Kimura, M., and Crow, J. F. (1964). The number of alleles that can be maintained in a finite population. Genetics 49 725.
King, J. L., and Jukes, T. H. (1969). Non-Darwinian evolution. Science 164 788.
Konigsberg, W. (1967). Subtractive Edman degradation. In Hirs, C. H. W. (ed.), Methods in Enzymology, Vol. XI: Enzyme Structure, Academic Press, New York, p. 461.
Kunkel, H. G., Ceppellini, R., Müller-Eberhard, U., and Wolf, J. (1957). Observations on the minor basic hemoglobin component in the blood of normal individuals and patients with thalassemia. J. Clin. Invest. 36 1615.
Marshall, R. E., Caskey, C. T., and Nirenberg, M. (1967). Fine structure of RNA codewords recognized by bacterial, amphibian, and mammalian transfer RNA. Science 155 820.
Matioli, G. T., and Niewisch, H. B. (1965). Electrophoresis of hemoglobin in single erythrocytes. Science 150 1824.
Maynard-Smith, J. (1970). Population size, polymorphism, and the rate of non-Darwinian evolution. Am. Naturalist 104 231.
Moore, S., and Stein, W. H. (1963). Chromatographic determination of amino acids by the use of automatic recording equipment. In Colowick, S. P., and Kaplan, N. O. (eds.), Methods in Enzymology, Vol. VI, Academic Press, New York, p. 819.
Mross, G. A., Doolittle, R. F., and Roberts, B. F. (1970). Gibbon fibrinopeptides: Identification of glycine-serine allelism at position B-3. Science 170 468.
Perutz, M. F., and Lehmann, H. (1968). Molecular pathology of human haemoglobin. Nature 219 902.
Perutz, M. F., Kendrew, J. C., and Watson, H. C. (1965). Structure and function of haemoglobin. II. Some relations between polypeptide chain configuration and amino acid sequence. J. Mol. Biol. 13 669.
Schapira, G., Benrubi, M., Maleknia, N., and Reibel, L. (1969). Hemoglobine de lapin: Une variante resultant d'un allelomorphisme et non d'un ambiguite: Hbα 29 Val→Leu. Biochim. Biophys. Acta 188 216.
Sick, K., Beale, D., Irvine, D., Lehmann, H., Goodall, P. T., and MacDougall, S. (1967). Haemoglobin GCopenhagen and Haemoglobin JCambridge. Two new β-chain variants of haemoglobin A. Biochim. Biophys. Acta 140 231.
Vogel, F. (1969). Point mutations and human hemoglobin variants. Humangenetik 8: 1.
Zuckerkandl, E., and Pauling, L. (1965). Evolutionary divergence and convergence in proteins. In Bryson, V., and Vogel, H. J. (eds.), Evolving Genes and Proteins, Academic Press, New York, p. 97.
Author information
Authors and Affiliations
Additional information
This investigation received support from grants to S.H.B., HD-02508-04 and K3-GM-6308-03, from the National Institutes of Health.
Rights and permissions
About this article
Cite this article
Boyer, S.H., Crosby, E.F., Noyes, A.N. et al. Primate hemoglobins: Some sequences and some proposals concerning the character of evolution and mutation. Biochem Genet 5, 405–448 (1971). https://doi.org/10.1007/BF00487132
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00487132