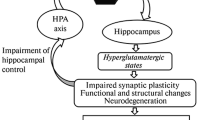
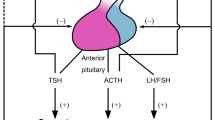
Summary
Hormones of the limbic-hypothalamicpituitary-adrenocortical (LHPA) system are much involved in central nervous system regulation. The major LHPA neuropeptides, corticotropin-releasing hormone (CRH), vasopressin (AVP) and corticotropin (ACTH) do not only coordinate the neuroendocrine response to stress, but also induce behavioral adaptation. Transcription and post-translational processing of these neuropeptides is regulated by corticosteroids secreted from the adrenal cortex after stimulation by ACTH and other pro-opiomelanocortin derived peptides. These steroids play a key role as regulators of cell development, homeostatic maintenance and adaptation to environmental challenges. They execute vitally important actions through genomic effects resulting in altered gene expression and nongenomic effects leading to altered neuronal excitability. Since excessive secretory activity of this particular neuroendocrine system is part of an acute stress response or depressive symptom pattern, there is good reason to suspect that central actions of these steroids and peptides are involved in pathophysiology determining the clinical phenotype, drug response and relapse liability.
This overview summarizes the clinical neuroendocrine investigations of the author and his collaborators, while they worked at the Department of Psychiatry in Mainz. The major conclusions from this work were: (1) aberrant hormonal responses to challenges with dexamethasone, ACTH or CRH are reflecting altered brain physiology in affective illness and related disorders; (2) hormones of the LHPA axis influence also nonendocrine behavioral systems such as sleep EEG; (3) physiologically significant interactions exist between LHPA hormones, the thyroid, growth hormone, gonadal and other neuroendocrine systems; (4) hormones of the LHPA axis constitute a bidirectional link between immunoregulation and brain activity; and (5) future psychiatric research topics such as molecular genetics of affective disorders, familial risk studies, drug response analysis and neurobiology of aging will benefit from extended knowledge of neural corticosteroid effects at a clinical, cellular, and molecular level.
Similar content being viewed by others
References
Aguilera G, Wynn PC, Harwood JP, Hanger RL, Millan MA, Grewe C, Catt KJ (1986) Receptor-mediated actions of corticotropin-releasing factor in pituitary gland and nervous system. Neuroendocrinology 43:79–88
Almeida OFX, Nikolarakis KE, Herz A (1988) Evidence for the involvement of endogenous opioids in the inhibition of luteinizing hormone by corticotropin-releasing factor. Endocrinology 122:1034–1041
Amsterdam JD, Maislin G, Winokur A, Kling M, Gold P (1987a) Pituitary and adrenocortical responses to the ovine corticotropin-releasing hormone in depressed patients and healthy volunteers. Arch Gen Psychiatry 44:775–781
Amsterdam JD, Marinelli DL, Arger P, Winokur A (1987b) Assessment of adrenal gland volume by computed tomography in depressed patients and healthy volunteers: a pilot study. Psychiatry Res 21:189–197
Arana GW, Workman RJ, Baldessarini RJ (1984) Association between low plasma levels of dexamethasone and elevated levels of cortisol in psychiatric patients given dexamethasone. Am J Psychiatry 141:1619–1620
Arana GW, Baldessarini RJ, Ornsteen M (1985) The dexamethasone suppression test for diagnosis and prognosis in psychiatry. Commentary and review. Ach Gen Psychiatry 42:1193–1204
Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM (1987) Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275
Banki CM, Bissette G, Arato M, O'Connor L, Nemeroff MS, Nemeroff CB (1987) CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry 144:7
Bardeleben U von, Holsboer F, Stalla GK, Müller OA (1985) Combined administration of human corticotropin-releasing factor and lysine vasopressin induces cortisol escape from dexamethasone suppression in healthy subjects. Life Sci 7:1613–1618
Bardeleben U von, Stalla GK, Müller OA, Holsboer F (1988a) Blunting of ACTH response to h-CRH in depressed patients is avoided by metyrapone pretreatment. Biol Psychiatry 24:782–786
Bardeleben U von, Wiedemann K, Stalla GK, Müller OA, Holsboer F (1988b) Exaggerated corticotrophic cell response to human corticotropin-releasing hormone in two patients during long-term carbamazepine treatment. Biol Psychiatry 24:331–335
Bardeleben U von, Lauer C, Wiedemann K, Holsboer F (1988c) Nocturnal sleep-endocrine effects of cortisol infusions in normal controls. Neuroendocrinol Lett 10:227
Bardeleben U von, Heuser I, Holsboer F (1989) Human CRH stimulation response during acute withdrawal and after medium-term abstention from alcohol abuse. Psychoneuroendocrinology (in press)
Bardeleben U von, Holsboer F (1989) Cortisol response to corticotropin releasing hormone in dexamethasone pretreated patients with depression. Neuroendocrinology (in press)
Beato M, Arnemann J, Chalepakis G, Slater E, Willmann T (1987) Gene regulation by steroid hormones. J Steroid Biochem 27:9–14
Berger M, Krieg C, Bossert S, Schreiber W, Von Zerssen D (1988) Past and present strategies of research on the HPA-axis in psychiatry. Acta Psychiatr Scand [Suppl] 77:112–125
Berkenbosch F, Van Oers J, Del Rey A, Tilders F, Besedovsky H (1987) Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science 238:524
Bernton EW, Beach JE, Holaday JW, Smallridge RC, Fein HG (1987) Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science 238:519–521
Besedovsky H, Del Rey A, Da Prada M, Burri R, Honegger C (1983) The immune response evokes changes in brain noradrenergic neurons. Science 221:564–565
Besedovsky H, Del Rey A, Sorkin E, Dinarello CA (1986) Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233:652–654
Beyer HS, Matta SG, Sharp BM (1988) Regulation of the messenger ribonucleic acid for corticotropin-releasing factor in the paraventricular nucleus and other brain sites of the rat. Endocrinology 123:2117–2123
Blundell JE (1984) Serotonin and appetite. Neuropharmacology 23:1537
Bohus B, De Wied D (1980) Pituitary-adrenal system hormones and adaptive behaviour. In: Chester-Jones I, Henderson IW (eds) General, comparative and clinical endocrinology of the adrenal cortex, vol 3. Academic Press, London, pp 256–347
Born J, Kern W, Bieber K, Fehm-Wolfsdorf G, Schiebe M, Fehm HL (1986) Night-time plasma cortisol secretion is associated with specific sleep stages. Biol Psychiatry 21:1415–1424
Breder C, Dinarello CA, Saper CB (1988) Interleukin-1 immunoreactive innervation of the human hypothalamus. Science 240:321
Briski KP, Quigley K, Meites J (1984) Endogenous opiate involvement in acute and chronic stress induced-changes in plasma LH concentration in the male rat. Life Sci 34:2485
Britton DR, Koob GF, Rivier J, Vale W (1982) Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci 31:363–367
Brown M, Fisher L, Spiess J, Rivier C, Rivier J, Vale W (1982) Corticoprotein-releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology 111:928–931
Carroll BJ, Martin FIr, Davies BM (1968) Resistance to suppression by dexamethasone of plasma 11-OHCS levels in severe depressive illness. Br Med J 3:285–287
Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James N, Kronfol Z, Lohr N, Steiner M, De Vigne JP, Young E (1981) A specific laboratory test for the diagnosis of melancholia. Arch Gen Psychiatry 38:15–22
Ceda GP, Davis RG, Hoffman AR (1987) Glucocorticoid modulation of growth hormone secretion in vitro. Evidence for a biphasic effect on GH-releasing hormone mediated release. Acta Endocrinol (Copenh) 114:465–469
Collins S, Caron MG, Lefkowitz RJ (1988) R2-adrenergic receptors in hamster smooth muscle cells are transcriptionally regulated by glucocorticoids. J Biol Chem 263:9067–9070
Cooper SJ (1986) Hyperphagic and anorectic effects of betacarbolines in a palatable food consumption test: comparisons with triazolam and quazepam. Eur J Pharmacol 120:257
Cooper SJ, Estall LB (1985) Behavioral pharmacology of food, water and salt intake in relation to drug actions at benzodiazepine receptors. Neurosci Biobehav Rev 9:5
Dave JR, Eiden LE, Karanian JW, Eskay RL (1986) Ethanol exposure decreases pituitary corticotropin-releasing factor binding, adenylate cyclase activity, proopiomelanocortin biosynthesis, and plasma beta-endorphin levels in the rat. Endocrinology 118:280–286
Eberwine JH, Jonassen JA, Evinger MJ, Roberts JL (1987) Complex transcriptional regulation by glucocorticoids and corticotropin releasing hormone of proopiomelanocortin gene expression in rat pituitary cultures. DNA 6:483–492
Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE (1983) Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res 278:332
Ehlers CL, Reed TK, Heriksen SJ (1986) Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology 42:467–474
Escamilla RF, Lisser H (1942) Simmonds' disease. A clinical study with review of the literature; differentiation from anorexia nervosa by statistical analysis of 595 cases, 101 of which were proved pathologically. J Clin Endocrinol 2:65–96
Eriksson E, Balldin J, Lindstedt G, Modigh K (1988) Growth hormone responses to the alpha2-adrenoceptor agonist guanfacine and to growth hormone releasing hormone in depressed patients and controls. Psychiatry Res 26:59–67
Estivariz FE, Carino M, Lowry PJ, Jackson S (1988) Further evidence that N-terminal pro-opiomelanocortin peptides are involved in adrenal mitogenesis. J Endocrinol 116:201–206
Fehm HL, Holl R, Späth-Schwalbe E, Born J, Voigt KH (1988) Ability of corticotropin releasing hormone to stimulate cortisol secretion independent from pituitary adrenocorticotropin. Life Sci 42:679–686
Felten DL, Ackerman KD, Wiegand SJ, Felten SY (1987) Noradrenergic sympathic innervation of the spleen. I. nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res 18:28–36
Fichter MM, Pirke KM, Holsboer F (1986) Weight loss causes neuroendocrine disturbances: experimental study in healthy starving subjects. Psychiatry Res 17:61–72
Fraser CM, Venter JC (1980) The sythesis of beta-adrenergic receptors in cultured human lung cells: induction by glucocorticoids. Biochem Biophys Res Commun 94:390–397
Ganong WF, Kramer N, Salmon J, Reid IA, Lovinger R, Scapagnini U, Boryczka AT, Shakelford R (1976) Pharmacological evidence for inhibition of ACTH secrection by a central adrenergic system in the dog. Neuroscience 1:167–174
Gelato MC, Pescovitz OH, Cassorla F, Loriaux L, Merriam GR (1984) Dose-response relationships for the effects of growth hormone-releasing factor-(1-44)-NH2 in young adult men and women. J Clin Endocrinol Metab 59:197–201
Gerken A, Holsboer F (1986) Cortisol and corticosterone response after syncorticotropin in relationship to dexamethasone suppressibility of cortisol. Psychoneuroendocrinology 11:185–194
Gerken A, Maier W, Holsboer F (1985) Weekly monitoring of dexamethasone suppression response in depression. Its relationship to change of body weight and psychopathology. Psychoneuroendocrinology 10:261–271
Gick GG, Zeytin FN, Brazeau P, Ling NC, Esch FS, Bancroft C (1984) Growth hormone-releasing factor regulates growth hormone mRNA in primary cultures of rat pituitary cells. Proc Natl Acad Sci USA 81:1553–1555
Gillies GE, Lowry PJ (1979) Corticotropin-releasing factor may be modulated by vasopressin. Nature 278:463–464
Gillies GE, Linton EA, Lowry PJ (1982) Corticotropin-releasing activity of the new CRF is potentiated several times by vasopressin. Nature 299:355–357
Gold PW, Chrousos G, Kellner C, Post R, Roy A, Augerinos P, Schulte H, Oldfield E, Loriaux DL (1984) Psychiatric implications of basic and clinical studies with corticotropin-releasing factor. Am J Psychiatry 141:619–627
Gold PW, Loriaux DL, Roy A, Kling MA, Clabrese JR, Kellner CH, Nieman LK, Post RM, Pickar D, Gallucci W, Augerinos P, Paul S, Oldfield EH, Cutler GB Jr, Chrousos GP (1986a) Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implic diagnostic implications. N Engl J Med 314:1329–1335
Gold PW, Gwirtsman H, Avgerinos PC, Nieman LK, Galluci WT, Kaye W, Jimerson D (1986b) Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. Pathophysiologic mechanisms in underweight and weight-corrected patients. The New Engl J Med 314:1335–1342
Greden JF, Gardner R, King D, Grunhaus L, Carroll BJ, Kronfol Z (1983) Dexamethasone suppression tests in anti-depressant treatment of melancholia — the process of normalization and test-retest reproducibility. Arch Gen Psychiatry 40:493–500
Guillaume V, Conte-Devolx B, Szafarczyk A, Malaval F, Pares-Herbute N, Grino M, Alonso G, Assenmacher I, Oliver C (1987) The corticotropin-releasing factor release in rat hypophysial portal blood is mediated by brain catecholamines. Neuroendocrinology 46:143–146
Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS (1985) Cortisol secretion in endogenous depression. II. Time related functions. Arch Gen Psychiatry 42:904–914
Härfstrand A, Fuxe K, Cintra A, Agnati LF (1986) Glucocorticoid receptor immunoreactivity in monoaminergic neurons of the rat brain. Proc Natl Acad Sci USA 83:9779–9783
Hauger RL, Milian MA, Catt KJ, Aguilera G (1987) Differential regulation of brain and pituitary corticotropin-releasing factor receptors by corticosterone. Endocrinology 120:1527–1533
Hermus AR, Pieters GF, Pesman GJ, Hofman J, Smals AG, Benraad TJ, Kloppenborg PW (1987) Escape from dexamethasone-induced ACTH and cortisol suppression by corticotrophin-releasing hormone: modulation effect of basal dexamethasone levels. Clin Endocrinol 136:67–74
Heuser I, Bardeleben U von, Boll E, Holsboer F (1988) Response of ACTH and cortisol to human corticotropin-releasing hormone after short-term abstention from alcohol abuse. Biol Psychiatry 24:316–321
Holsboer F (1983) Prediction of clinical course by dexamethasone suppression test (DST) response in depressed patients — physiological and clinical construct validity of the DST. Pharmacopsychiatry 16:186–191
Holsboer F (1985) Adrenocortical steroid secretion and 11-ß-steroidhydroxylase activity after pituitary-adrenocortical probes in depression. Psychiatr Med 3:65–78
Holsboer F, Bender W, Benkert O, Klein HE, Schmauss M (1980) Diagnostic value of dexamethasone suppression test in depression. Lancet 1:706
Holsboer F, Doerr HG, Sippell WG (1982a) Blunted aldosterone response for dexamethasone in female patients with endogenous depression. Psychoneuroendocrinology 7:155–162
Holsboer F, Liebl R, Hofschuster E (1982b) Repeated dexamethasone suppression test during depressive illness. Normalization of test result compared with clinical improvement. J Affective Disord 4:93–101
Holsboer F, Steiger A, Maier W (1983) Four cases of reversion to abnormal dexamethasone suppression test response as indicator of clinical relapse: a preliminary report. Biol Psychiatry 18:911–916
Holsboer F, Bardeleben U von, Gerken A, Stalla GK, Müer OA (1984a) Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor (h-CRF) in depression. N Engl J Med 311:1127
Holsboer F, Müer OA, Doerr HG, Sippell WG, Stalla GK, Gerken A, Steiger A, Boll E, Benkert O (1984b) ACTH and multisteroid responses to corticotropin-releasing factor in depressive illness: relationship to multisteroid responses after ACTH stimulation and dexamethasone suppression. Psychoneuroendocrinology 9:147–160
Holsboer F, Gerken A, Steiger A, Benkert O, Müer OA, Stalla GK (1984c) Corticotropin-releasing factor induced pituitary-adrenal responses in depression. Lancet 1:55
Holsboer F, Haack D, Gerken A, Vecsei P (1984d) Plasma dexamethasone concentrations and differential glucocorticoid suppression response in depressives and controls. Biol Psychiatry 19:281–291
Holsboer F, Gerken A, Steiger A, Fass V (1984e) The mean 14.00h–17.00h plasma cortisol concentration and its relationship to the 1 mg dexamethasone suppression response in depressives and controls. Acta Psychiatr Scand 69:383–390
Holsboer F, Gerken A, Stalla GK, Müller OA (1985a) ACTH, cortisol and corticosterone output after ovine corticotropin-releasing factor challenge during depression and after recovery. Biol Psychiatry 20:276–286
Holsboer F, Gerken A, Bardeleben U von, Grimm W, Stalla GK, Müller OA (1985b) Relationship between pituitary responses to human corticotropin-releasing factor and thyrotropin-releasing hormone in depressives and normal controls. Eur J Pharmacol 110:153–154
Holsboer F, Gerken A, Bardeleben U von, Grimm W, Beyer H, Müller OA, Stalla GK (1986a) Human corticotropin-releasing hormone in depression. Biol Psychiatry 21:609–611
Holsboer F, Philipp M, Steiger A, Gerken A (1986b) Multisteroid analysis after DST in depressed patients — a controlled study. J Affective Disord 10:241–249
Holsboer F, Wiedemann K, Boll E (1986c) Shortened dexamethasone half-life time in depressed dexamethasone nonsuppressors. Arch Gen Psychiatry 43:813–815
Holsboer F, Wiedemann K, Gerken A, Boll E (1986d) The plasma dexamethasone variable in depression — test retest studies and early biophase kinetics. Psychiatry Res 17:97–103
Holsboer F, Gerken A, Stalla GK, Müller OA (1987a) Blunted aldosterone and ACTH release after human corticotropin-releasing hormone in depression. Am J Psychiatry 144:229–231
Holsboer F, Bardeleben U von, Buller R, Heuser I, Steiger A (1987b) Stimulation response to corticotropin-releasing hormone (CRH) in patients with depression, alcoholism and panic disorder. Horm Metab Res 16:80–88
Holsboer F, Bardeleben U von, Wiedemann K, Müller OA, Stalla GK (1987c) Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression — implications for pathophysiology of DST nonsuppression. Biol Psychiatry 22:228–234
Holsboer F, Bardeleben U von, Steiger A (1988a) Effects of intravenous corticotropin-releasing hormone upon sleep-related growth hormone surge and sleep-EEG in man. Neuroendocrinology 48:62–68
Holsboer F, Stalla GK, von Bardeleben U, Hamman K, Mülller H, Müller OA (1988b) Acute adrenocortical stimulation by recombinant gamma interferon in human controls. Life Sci 42:1–5
Hotta M, Shibasaki T, Masuda A, Imaki T, Demura H, Ling N, Shizuma K (1986) The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. Endocrinol Metab 62:319–324
Hotta M, Shibasaki T, Masuda A, Imaki T, Sugino N, Demura H, Ling N, Shizume K (1988) Effect of human growth hormone-releasing hormone on GH secretion in Cushing's syndrome and non-endocrine disease patients treated with glucocorticoids. Life Sci 42:979–984
Hu ZY, Bourreau E, Jung-Testas I, Robel P, Baulieu E-E (1987) Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci USA 84:9215–9219
Hullin RP, Levell MJ, O'Brien MJ, Toumba KJ (1981) Inhibition of in vitro production of aldosterone by manic depressive sera. Br J Psychiatry 138:373–380
Insel TR, Ninan PT, Aloi Jimerson DC, Skolnick P, Paul SM (1984) A benzodiazepine receptor-mediated model of anxiety. Arch Gen Psychiatry 41:741
Irwin MR, Vale W, Britton KT (1987) Central corticotropin-releasing factor suppresses natural killer cytotoxicity. Brain Behav Immun 1:81–87
Jaeckle RS, Kathol RG, Lopez JF, Meller WH, Krummel SJ (1987) Enhanced adrenal sensitivity to exogenous cosyntropin (ACTH-alpha 1-24) stimulation in major depression. Relationship to dexamethasone suppression test results. Arch Gen Psychiatry 44:233–240
Kamilaris TC, Debold CR, Pavlou SN, Island DP, Hoursanidis A, Orth DN (1987) Effect of altered thyroid hormone levels on hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab 65:994–999
Kaye WH, Gwirtsman HE, George DT, Ebert MH, Jimerson DC, Tomai TP, Chrousos GP, Gold PW (1987) Elevated cerebrospinal fluid levels of immunoreactive corticotropin-releasing hormone in anorexia nervosa: relation to state of nutrition, adrenal function and intensity of depression. J Clin Endocrinol Metab 64:203–208
Krishnan KRR, Manepalli AN, Ritchie JC, Rayasam K, Melville ML, Daughtry G, Thormer MO, Rivier JE, Vale WW, Nemeroff CB, Carroll BJ (1988) Growth hormonereleasing factor stimulation test in depression. Am J Psychiatry 145:90–92
Lambert JJ, Peters JA, Cottrell GA (1987) Actions of synthetic and endogenous steroids on the GABAA receptor. Trends Pharmacol Sci 8:224–227
Lee SW, Tsou A-P, Chan H, Thomas J, Petrie K, Eugui EM, Allison AC (1988) Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci USA 85:1204–1208
Leibowitz SF, Hammer NJ, Chang K (1983) Feeding behavior induced by central norepinephrine injection is attenuated by discrete lesions in the hypothalamic paraventricular nucleus. Pharmacol Biochem Behav 19:945
Lesch KP, Laux G, Erb A, Pfüller H, Beckmann H (1987) Attenuated growth hormone response to growth hormone-releasing hormone in major depressive disorder. Biol Psychiatry 22:1491–1495
Lesch KP, Laux G, Schulte HM, Pfüller H, Beckmann H (1988) Corticotropin and cortisol response to human CRH as a probe for HPA system integrity in major depressive disorder. Psychiatry Res 24:25–34
Leseney AM, Benmiloud M, Beford N, Befort JJ (1987) In vitro evidence that hypothyroidism modifies glucocorticoid receptors. Mol Cell Biol 52:1–10
Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, Copinschi G, Van Canter E (1987) 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab 65:141–152
Lopez-Calderon A, Ariznavarreta C, Calderon MD, Tresguerres JAF (1987) Gonadotropin inhibition during chronic stress: role of the adrenal gland. J Steroid Biochem 27:609–614
Lumpkin MD, Samson WK, McCann SM (1987) Arginine vasopression as a thyrotropin-releasing hormone. Science 235:1070–1073
Lytras N, Grossman A, Perry L, Tomlin S, Wass J, Coy D, Schally A, Rees L, Besser G (1984) Corticotropin-releasing factor: responses in normal subjects and patients with disorders of the hypothalamus and pituitary. Clin Endocrinol 20:71–84
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM (1986) Steroid hormone metabolites are barbituratelike modulators of the GABA receptors. Science 231:1004–1007
Matussek N, Ackenheil M, Hippius H, Müller F, Schröder HT, Schultes H, Wasilewski B (1980) Effect of clonidine on growth hormone release in psychiatric patients and controls. Psychiatry Res 2:25–36
McEwen BS, De Kloet ER, Rostene W (1986) Adrenal steroid receptors and actions in the nervous system. Physiol Rev 66:1121–1188
Meaney MJ, Aitken DH, Sapolsky RM (1987) Thyroid hormones influence the development of hippocampal glucocorticoid receptors in the rat: a mechanism for the effects of postnatal handling on the development of the adrenocortical stress response. Neuroendocrinology 45:278–283
Mobley PL, Sulser F (1980) Adrenal corticoids regulate sensitivity of noradrenaline receptor-coupled adenylate cyclase in brain. Nature 286:608–609
Mochly-Rosen D, Chang F-H, Cheever L, Kim M, Diamond I, Gordon AD (1988) Chronic ethanol causes heterologous desensitization of receptors by reducing alphas messenger RNA. Nature 333:304–305
Morley JE, Levine AS (1982) Corticotrophin releasing factor, grooming and ingestive behavior. Life Sci 31:1459–1464
Mortola JF, Liu JH, Gillin JC, Rasmussen DD, Yen SSC (1987) Pulsatile rhythms of adrenocorticotropin (ACTH) and cortisol in women with endogenous depression: evidence for increased ACTH pulse frequency. J Clin Endocrinol Metab 65:962
Nakagawa K, Ishizuka I, Obara T, Matsubara M, Akikawa K (1987) Dichotomic action of glucocorticoids on growth hormone secretion. Acta Endocrinol (Copenh) 116:165–171
Nemeroff CB, Widerlöv E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CS, Loosen PT, Vale W (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344
Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M (1988) Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 45:577–579
Nyborg JK, Nguyen AP, Spindler SR (1984) Relationship between thyroid and glucocorticoid hormone receptor occupancy, growth hormone gene transcription, and mRNA. J Biol Chem 259:12377–12381
Okajima T, Hertting G (1986) The possible involvement of cytochrome P-450 mono-oxygenase in AVP-induced ACTH secretion. Horm Metab Res 18:281–282
Pater MM, Hughes GA, Hyslop DE, Nakshatri H, Pater A (1988) Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature 335:832
Plotsky PM, Otto S, Sutton S (1987) Neurotransmitter modulation of corticotropin releasing factor secretion into the hypophysial-portal circulation. Life Sci 41:1311–1317
Post RM, Weiss SRB, Rubinow DR (1988) Recurrent affective disorders: lessons from limbic kindling. Curr Top Neuroendocrinol 8:91–115
Reisine T, Hoffman A (1983) Desensitization of corticotropin releasing factor receptors. Biochem Biophys Res Commun 111:919–925
Reisine T, Affolter M-U, Rougon G, Barbet J (1986) New insights into the molecular mechanisms of stress. Trends Neurosci 9:574–579
Reul JM, Kloet ER de (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505–2511
Reul JM, Bosch FR van den, Kloet ER de (1987) Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. J Endocrinol 115:459–467
Ringold GM (1985) Steroid hormone regulation of gene expression. Am Rev Pharmacol Toxicol 25:529–566
Risch SC, Golshan S, Rapaport MH, Dupont R, Outenreath R, Gillin JC, Janowsky DS (1988) Neuroendocrine effects of intravenous ovine corticotropin-releasing factor in affective disorder patients and normal controls. Biol Psychiatry 23:755–758
Rivier C, Vale W (1984) Corticotropin-releasing factor (CRF) acts centrally to inhibit growth hormone secretion in the rat. Endocrinology 114:2409–2411
Rivier C, Brun T, Vale W (1984) Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther 229:127–131
Rivier C, Rivier J, Vale W (1986) Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science 231:607–609
Robel P, Bourreau E, Corpéhot C, Dang DC, Halberg F, Clarke C, Haug M, Schlegel ML, Synguelakis M, Vourch C, Baulieu EE (1987) Neuro-steroids: 3-beta-hydroxy-5-derivatives in rat and monkey brain. J Steroid Biochem 27:649–655
Robinson BG, Emanuel RL, Frim DM, Majzoub JA (1988) Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Nat Acad Sci USA 85:5244–5248
Rousseau GG, Eliard PH, Barlow JW, Lemaigre FP, Lafontaine DA, De Nayer P, Economidis IV, Formstecher P, Idziorek T, Mathy-Hartert M, Voz MLJ, Belayew A, Martial JA (1987) Approach to the molecular mechanisms of the modulation of growth hormone gene expression by glucocorticoid and thyroid hormones. J Steroid Biochem 27:149–158
Ruckebusch Y, Malbert CH (1985) Stimulation and inhibition of food intake in sheep by centrally-administered hypothalamic releasing factors. Life Sci 38:929–934
Salata RA, Jarrett DB, Verbalis JG, Robinson AG (1988) Vasopressin stimulation of adrenocorticotropin hormone (ACTH) in humans. J Clin Invest 81:766–774
Sapolsky RM (1983) Individual differences in cortisol secretory patterns in the wild baboon: role of negative feedback sensitivity. Endocrinology 113:2263–2267
Sapolsky RM, Krey LC, McEwen BS (1984) Glucocorticoidsensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci USA 81:6174–6177
Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W (1987) Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238:522
Sapolsky RM, Packan DR, Vale WW (1988) Glucocorticoid toxicity in the hippocampus: in vitro demonstration. Brain Res 453:367–371
Scheidereit C, Beato M (1984) Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci USA 81:3029–3033
Schleifer SJ, Keller SE, Meyerson AT, Raskin MJ, Davis KL, Stein M (1984) Lymphocyte function in major depressive disorder. Arch Gen Psychiatry 41:484–486
Schwartz J, Vale W (1988) Dissociation of the adrenocorticotropin secretory responses to corticotropin-releasing factor (CRF) and vasopressin or oxytocin by using a specific cytotoxic analog of CRF. Endocrinology 122:1695–1700
Shibahara S, Morimoto Y, Furutani Y, Notake M, Takahashi H, Shimizu S, Horikawa S, Numa S (1983) Isolation and sequence analysis of the human corticotropin-releasing factor precursor gene. EMBO J 2:775–779
Sirinatsinghji DJS, Rees LH, Rivier J, Vale W (1983) Corticotropin-releasing factor is a potent inhibitor of sexual receptivity in the female rat. Nature 305:232–235
Smith EM, Morrill AC, Meyer WJ III, Blalock JE (1986) Corticotropin-releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. Nature 321:881–882
Sorenson D (1987) A role for glucocorticoids in the polyphosphoinositide second messenger system. Med Hypotheses 22:309–319
Spiess J, Rivier J, Rivier C, Vale W (1981) Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA 78:6517–6521
Stalla GK, Hartwimmer J, Schopohl J, von Werder K, Müller OA (1986) Intravenous application of ovine and human corticotropin releasing factor (CRF): ACTH, cortisol and CRF levels. Neuroendocrinology 42:1–5
Steiger A, Herth T, Holsboer F (1987) Sleep-EEG and the secretion of cortisol and human growth hormone in normal controls. Acta Endocrinol (Copenh) 116:36–42
Steiger A, Bardeleben U von, Herth T, Holsboer F (1989) Sleep-EEG and nocturnal secretion of cortisol and human growth hormone in male patients with endogenous depression before treatment and after recovery. J Affective Disord 16:189–195
Suemaru S, Hashimoto K, Hattori T, Inoue H, Kageyama J, Ota Z (1986) Starvation-induced changes in rat brain corticotropin-releasing factor (CRF) and pituitary-adrenocortical response. Life Sci 39:1161
Swartz GM, Dunner FJ (1982) Dexamethasone suppression testing of alcoholics. Arch Gen Psychiatry 39:1309–1312
Tabakoff B, Hoffman PL, Lee JM, Saito T, Willard B, De Leon-Jones F (1988) Differences in platelet enzyme activity between alcoholics and nonalcoholics. N Engl J Med 318:134–139
Thomas AP, Alexander J, Williamson JR (1984) Relationship between inositol polyphosphate production and the increase of cytosolic-free Ca 2+ induced by vasopressin in isolated hepatocytes. J Biol Chem 259:5574–5584
Todd K, Lightman SL (1987) Vasopressin activation of phosphalidylinositol metabolism in rat anterior pituitary in vitro and its modification by changes in the hypothalamo-pituitary-adrenal axis. Neuroendocrinology 45:212–218
Tuomisto J, Mannisto P (1985) Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev 37:249–332
Uehara A, Gillis S, Arimura A (1987) Effects of interleukin-1 on hormone release from normal rat pituitary cells in primary culture. Neuroendocrinology 45:343–347
Valcavi R, Jordan V, Dieguez C, John R, Manicardi E, Portiolo I, Rodriguez-Arnao MD, Gomez-Pan A, Hall R, Scanlon MF (1986) Growth hormone responses to GRF 1–29 in patients with primary hypothyroidism before and during replacement therapy with thyroxine. Clin Endocrinol 24:693–698
Vale W, Vaughn J, Smith M, Yamamoto G, Rivier C (1983) Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, cathecholamines, neurohypophysial peptides and other substances on cultured corticotrophic cells. Endocrinology 113:1121
Wand GS, Eipper BA (1987) Effect of chronic secretagogue exposure on proadrenocorticotropin/endorphin production and secretion in primary cultures of rat anterior pituitary. Endocrinology 120:953–961
Weiss SRB, Post RM, Gold PW, Chrousos G, Sullivan TL, Walker D, Pert A (1986) CRF-induced seizures and behavior: interaction with amygdala kindling. Brain Res 372:345
Wiedemann K, Holsboer F (1987) Plasma dexamethasone levels in depression: oral versus i.v. administration. Psychiatry Res 20:83–85
Wolowski BM, Smith EM, Meyer WJ, Fuller GM, Blalock JE (1985) Corticotropin-releasing activity of monokines. Science 230:1035–1037
Young EA, Akil H (1985) Corticotropin-releasing factor stimulation of adreno-corticotropin and beta-endorphin release: effects of acute and chronic stress. Endocrinology 117:23–30
Zatz M, Reisine TD (1985) Lithium induces corticotropin secretion and desensitization in cultured anterior pituitary cells. Proc Natl Acad Sci USA 82:1286–1290
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holsboer, F. Psychiatric implications of altered limbic-hypothalamic-pituitary-adrenocortical activity. Eur Arch Psychiatr Neurol Sci 238, 302–322 (1989). https://doi.org/10.1007/BF00449812
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00449812