Abstract
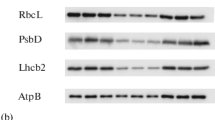
Expression of nuclear genes involved in plastidogenesis is known to be controlled by light via phytochrome. Examples are the small subunit (SSU) of ribulose-1,5-bisphosphate carboxylase and the light harvesting chlorophyll a/b binding protein of photosystem II (LHCP). In the present study we show that, beside phytochrome, the integrity of the plastid is essential for the expression of the pertinent nuclear genes as measured at the level of translatable mRNA. When the plastids are severely damaged by photooxidation in virtually carotenoid-free mustard (Sinapis alba L.) seedling cotyledons (made carotenoid-free by the application of Norflurazon, NF), almost no SSU, no SSU precursor, LHCP and LHCP precursor can be detected by immunological assays, and almost no translatable mRNA of SSU and LHCP can be found, although the levels and rates of phytochrome-mediated syntheses of representative cytoplasmic, mitochondrial and glyoxisomal enzymes are not adversely affected and morphogenesis of the mustard seedling proceeds normally (Reiß et al. 1983; Planta 159, 518–528). Norflurazon per se has no effect on the amount of translatable mRNA of SSU and LHCP as shown by irradiation of NF-treated seedlings with far-red light (FR) which strongly activates phytochrome but does not cause photooxidation in the plastids. It is concluded that a signal from the plastid is required to allow the phytochrome-mediated appearance of translatable mRNA for SSU and LHCP. Seedlings not treated with NF show a higher level of translatable mRNALHCP in red light (RL) compared to FR, whereas the mRNASSU levels are the same in RL and FR. These facts indicate that the level of translatable mRNALHCP is adversely affected if the apoprotein is not incorporated into the thylakoid membrane.
Similar content being viewed by others
Abbreviations
- FR:
-
far-red light (3.5 W m-2)
- LHCP:
-
light harvesting chlorophyll a/b binding protein of photosystem II
- LSU:
-
large subunit of RuBPCase
- NF:
-
Norflurazon
- RL:
-
red light (6.8 W m-2)
- RuBPCase:
-
ribulose-1,5-bisphosphate carboxylase (EC 4.1.1.39)
- SSU:
-
small subunit of RuBPCase
- WL:
-
white light (28 W m-2)
References
Apel, K. (1979) Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur. J. Biochem. 97, 183–188
Apel, K., Kloppstech, K. (1980) The effect of light on the biosynthesis of the light-harvesting chlorophyll a/b protein. Evidence for the requirement of chlorophyll a for the stabilization of the apoprotein. Planta 150, 426–430
Bennett, J. (1981) Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur. J. Biochem. 118, 61–70
Blair, G.E., Ellis, R.J. (1973) Protein synthesis in chloroplasts. I. Light driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim. Biophys. Acta 319, 223–234
Bonner, W.M., Laskey, R.A. (1974) A film detection method for tritium labelled proteins and nucleic acids in polyacrylamide gels. Eur. J. Biochem. 46, 83–88
Bradford, M.J. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Brüning, K., Drumm, H., Mohr, H. (1975) One the role of phytochrome in controlling enzyme levels in plastids. Biochem. Physiol. Pflanz 168, 141–156
Cashmore, A.R. (1979) Reteration frequency of the gene coding for the small subunit of ribulose-1,5-bisphosphate carboxylase. Cell 17, 383–388
Coen, D.M., Bedbrook, J.R., Bogorad, L., Rich, A. (1977) Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc. Natl. Acad. Sci. USA 74, 5487–5491
Dobberstein, B., Blobel, G., Chua, N.-H. (1977) In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 74, 1082–1085
Drumm-Herrel, H., Bergfeld, R., Mohr, H. (1984) Photooxidative destruction of chloroplasts and its consequences for anthocyanin synthesis. Proc. Indian Acad. Sci. 93, 245–251
Fairbanks, G., Steck, T.L., Wallach, D.F.H. (1971) Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10, 2606–2617
Fourcroy, P., Klein-Eude, D., Lambert, C. (1985) Phytochrome control of gene expression in radish seedlings. II. Far-red light mediated appearance of the ribulose 1,5-bisphosphate carboxylase and the mRNA for its small subunit. Plant Sci. Lett. 37, 235–244
Frosch, S., Bergfeld, R., Mohr, H. (1976) Light control of plastogenesis and ribulosebisphosphate carboxylase levels in mustard seedling cotyledons. Planta 133, 53–56
Frosch, S., Jabben, M., Bergfeld, R., Kleinig, H., Mohr, H. (1979) Inhibition of carotenoid biosynthesis by the herbicide SAN 9789 and its consequences for the action of phytochrome on plastogenesis. Planta 145, 497–505
Goldthwaite, J.J., Bogorad, L. (1971) A one-step method for the isolation and determination of leaf ribulose-1,5-diphosphate carboxylase. Anal. Biochem. 41, 57–66
Gottmann, K., Schäfer, E. (1982) In vitro synthesis of phytochrome apoprotein directed by mRNA from light and dark grown Avena seedlings. Photochem. Photobiol. 35, 521–525
Harpster, M.H., Mayfield, S.P., Taylor, W.C. (1984) Effects of pigment-deficient mutants on the accumulation of photosynthetic proteins in maize. Plant Mol. Biol. 3, 59–71
Highfield, P.E., Ellis, R.J. (1978) Synthesis and transport of the small subunit of chloroplast ribulose bisphosphate carboxylase. Nature 271, 420–424
Kreuz, K., Kleinig, H. (1984) Synthesis of prenyl lipids in cells of spinach leaf. Compartimentation of enzymes for formation of isopentenyl diphosphate. Eur. J. Biochem. 141, 531–535
Kung, S.D., Thornber, J.P., Wildman, S.G. (1972) Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 24, 185–188
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 227, 680–685
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951) Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275
Mayfield, S.P., Taylor, W.C. (1984) Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur. J. Biochem. 144, 79–84
Mohr, H. (1966) Untersuchungen zur phytochrominduzierten Photomorphogenese des Senfkeimlings (Sinapis alba L.) Z. Pflanzenphysiol. 54, 63–83
Mohr, H. (1972) Lectures on photomorphogenesis. Springer, Berlin Heidelberg New York
Oelmüller, R., Mohr, H. (1985) Carotenoid composition in milo (Sorghum vulgare) shoots as affected by phytochrome and chlorophyll. Planta 164, 390–395
Occhterlony, O. (1968) Handbook of immunodiffusion and immunoelectrophoresis. Ann Arbor Science Publishers, Ann Arbor, Mich.
Pelham, H.R.B., Jackson, R.J. (1976) An efficient mRNA-dependent translation system from reticulocyte lysates. Eur. J. Biochem. 67, 247–256
Reiß, T., Bergfeld, R., Link, G., Thien, W., Mohr, H. (1983) Photooxidative destruction of chloroplasts and its consequences of cytocolic enzyme levels and plant development. Planta 159, 518–528
Roscoe, T.J., Ellis, R.J. (1982) Two-dimensional gel-electrophoresis of chloroplast proteins. In: Methods in chloroplast molecular biology, pp. 1015–1028, Edelman, M., Hallick, R.B., Chua, N.-H., eds. Elsevier Biomedical Press, Amsterdam New York Oxford
Schmidt, G.W., Bartlett, S.G., Grossman, A.R., Cashmore, A.R., Chua, N.-H. (1981) Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J. Cell Biol. 91, 468–478
Silverthorne, J., Tobin, E.M. (1984) Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc. Natl. Acad. Sci. USA 81, 1112–1116
Slovin, J.P., Tobin, E.M. (1982) Synthesis and turnover of the light-harvesting chlorophyll a/b-protein in Lemma gibba grown with intermittent red light: possible translational control. Planta 154, 465–472
Thornber, J.P. (1975) Chlorophyll-proteins: light-harvesting and reaction center components of plants. Annu. Rev. Plant Physiol. 26, 127–158
Tobin, E.M. (1981) Phytochrome-mediated regulation of messenger RNAs for the small subunit of ribulose-1,5-bisphosphate carboxylase and the light-harvesting chlorophyll a/b protein in Lemna gibba. Plant Mol. Biol. 1, 35–51
Towbin, H., Staehelin, T., Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oelmüller, R., Mohr, H. Photooxidative destruction of chloroplasts and its consequences for expression of nuclear genes. Planta 167, 106–113 (1986). https://doi.org/10.1007/BF00446376
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00446376