Abstract
-
1.
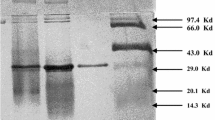
When growing with cyclodextrins, Klebsiella pneumoniae M 5 al produces extracellular cyclodextrin glucanotransferase in amounts comparable to those obtained during the growth with potato starch.
-
2.
Intracellular cyclodextrin glucanotransferase-activity was demonstrated to be present in the homogenates of cells grown with cyclodextrins. In addition, an amylomaltase-like enzyme and the maltodextrin phosphorylase could be pointed out. The cyclodextrins are metabolized to glucose-1-phosphate and glucose by the concerted actions of these three enzymes. paraGlucose-1-phosphate is liberated from cyclohexaamylose by the actions of purified cyclodextrin glucanotransferase and purified maltodextrin phosphorylase. The liberation of the sugar phosphate is increased fivefold by addition of glucose as an acceptor. This sugar, however, retards the formation of glucose-1-phosphate from the cyclic compound by the enzymes of the cell extract: In the presence of glucose the amylomaltase is incapable of synthesizing substrates for the phosphorylase from maltose. This experimental result clearly demonstrates that the amylomaltase is involved in the disproportionation of maltosaccharides arising from the cyclodextrins.
-
3.
A NADP+-specific glucose dehydrogenase was demonstrated to be present in the cell extracts. This enzyme, which is activated by ADP, may control the energy-depending pool of free glucose. Glucose originates from the disproportionation of maltosaccharides catalyzed by the glucanotransferases.
-
4.
A glucose-1-phosphate-hydrolysing phosphatase, which is shown to be present in the cell extract, seems to be without physiological significance for the metabolism of the cyclodextrins.
-
5.
Preliminary permeation studies make it probable that the cyclodextrins are transported into the cells as such and degraded only within the cells.
-
6.
A scheme for the metabolism of cyclodextrins in Klebsiella pneumoniae M 5 al is proposed.
Zusammenfassung
-
1.
Klebsiella pneumoniae M 5 al wächst mit Cyclodextrinen gleich gut wie mit Glucose. Die mit den cyclischen Kohlenstoffquellen synthetisierte Menge an extracellulärer Cyclodextrin-Glucanotransferase entspricht derjenigen, wie sie bei Wachstum mit Kartoffelstärke produziert wird.
-
2.
Im Zellextrakt der mit Cyclodextrinen gewachsenen Zellen kann intracelluläre Cyclodextrin-Glucanotransferase festgestellt werden. Daneben lassen sich ein amylomaltase-ähnliches Enzym und die Maltodextrin-Phosphorylase nachweisen. Diese drei Enzyme sind im wesentlichen für den Abbau der Cyclodextrine verantwortlich.
Gereinigte Cyclodextrin-Glucanotransferase und gereinigte Maltodextrin-Phosphorylase setzen aus Cyclohexaamylose Glucose-1-Phosphat frei. Während in diesem System die Geschwindigkeit der Glucose-1-Phosphat-Freisetzung durch Zugabe des Akzeptors Glucose verfünffacht wird, verzögert Glucose die Bildung von Glucose-1-Phosphat durch die Enzyme des Zellextraktes: Die Amylomaltase ist in Gegenwart von Glucose unfähig, Substrate für die Phosphorylase aus Maltose aufzubauen. Aufgrund dieser experimentellen Befunde kann die Beteiligung des Enzyms an der Disproportionierung der durch Linearisierung der Cyclodextrine entstehenden Maltosaccharide als sicher gelten.
-
3.
Das Zellhomogenat enthält eine durch ADP aktivierbare NADP+-spezifische Glucose-Dehydrogenase. Dieses Enzym könnte den energie-abhängigen Pool an freier Glucose kontrollieren. Glucose entsteht bei der Disproportionierung der Maltosacchaide durch die Glucanotransferasen.
-
4.
Eine im Zellextrakt nachzuweisende Glucose-1-Phosphat spaltende Phosphatase scheint keine physiologische Bedeutung beim Metabolismus der Cyclodextrine zu haben.
-
5.
Orientierende Permeationsuntersuchungen machen es wahrscheinlich, daß die Cyclodextrine als solche in die Zelle transportiert und erst innerhalb der Zelle linearisiert werden.
-
6.
Anhand der experimentellen Befunde wird ein Schema für den Metabolismus der Cyclodextrine durch Klebsiella pneumoniae M 5 al gegeben.
Similar content being viewed by others
Abbreviations
- Amylomaltase oder 1,4-α-d-glucan:
-
1,4-α-glucan-4-α-glycosyltransferase (EC 2.4.1.25)
- Cyclodextrin-Glucanotransferase oder 1,4-α-d-glucan:
-
4-α-(1,4-α-glucano)-transferase (cyclizing) (EC 2.4.1.19)
- Cyclodextrinase oder cyclic dextrin:
-
dextrin hydrolase (decyclizing) (EC 3.2.1.54)
- Glucose-Dehydrogenase oder β-d-glucose:
-
NAD(P)-1-oxidoreductase (EC 1.1.1.47)
- Maltodextrin-Phosphorylase oder 1,4-α-d-glucan:
-
orthophosphate glucosyltransferase (EC 2.4.1.1)
- (EC 3.1.3.1) bzw (EC 3.2.3.2):
-
Phosphatase oder orthophosphoric monoester phosphohydrolase
- CGT:
-
Cyclodextrin-Glucanotransferase
- Glc-1-P:
-
α-d-Glucopyranose-1-phosphorsäure
- Glc-1,6-DiP:
-
α-d-Glucopyranose-1,6-diphosphorsäure
- pNPP:
-
p-Nitrophenylphosphat
- Tris:
-
Tris(hydroxymethyl)-aminomethan
Literatur
Beisenherz, G., Boltze, H. J., Bücher, T., Czok, R., Garbade, K. H., Meyer-Arendt, E., Pfleiderer, G.: Diphosphofructosealdolase, Phosphoglyceraldehyd-dehydrogenase, Milchsäuredehydrogenase, Glycerophosphat-dehydrogenase und Pyruvatkinase aus Kaninchenmuskulatur in einem Arbeitsgang. Z. Naturforsch. 8b, 555–577 (1953)
Bender, H.: Cyclodextrin-Glucanotransferase von Klebsiella pneumoniae 1. Synthese, Reinigung und Eigenschaften des Enzyms von Klebsiella pneumoniae M 5 al. Arch. Microbiol. 111, 271–282 (1977)
Bender, H., Wallenfels, K.: Untersuchungen and Pullulan. II. Spezifischer Abbau durch ein bakterielles Enzym. Biochem. Z. 334 79–95 (1961)
Ben-Gershom, E., Leibowitz, J.: Enzymic degradation of the Schardinger dextrins. Enzymologia 20, 133–147 (1958)
Boeltz, F.: 1,4-α-Glucan-Phosphorylase aus K. pneumoniae M 5 al. Reinigung und Eigenschaften. Diplomarbeit, Universität Freiburg (1975)
Bolton, P. G., Dean, A. C. R.: Phosphatase synthesis in Klebsiella aerogenes growing in continuous culture. Biochem. J. 127, 87–96 (1972)
Brümmer, W., Ebeling, W.: Eigenschaften und Anwendung der Glucose-Dehydrogenase. Kontakte (Merck) 3–7 (1976)
DePinto, J. A., Campbell, L. L.: Purification and properties of the cyclodextrinase of Bacillus macerans. Biochemistry 7, 121–125 (1968a)
DePinto, J. A., Campbell, L. L.: Pattern of action of the amylase and the cyclodextrinase of Bacillus macerans. Arch. Biochem. Biophys. 125, 253–258 (1968b)
Essig, U.: Amylomaltase aus K. pneumoniae 1009/III. Diplomarbeit, Universität Freiburg (1976)
Fishman, W. H., Lerner, F.: A method for estimating serum acid phosphatase of prostatic origin. J. biol. Chem. 200, 89–97 (1953)
French, D.: The Schardinger dextrins. Advanc. Carbohydr. Chem. 12 189–260 (1957)
Häselbarth, V. Schulz, G. V., Schwinn, H.: Untersuchungen über Amylomaltase. II. Molekulare Konstanten und Wirkungsweise des Enzyms. Biochim. biophys. Acta (Amst.) 227, 296–312 (1971)
Lane, A. G., Pirt, S. J.: Production of cyclodextrin glycosyltransferase by batch and chemostat culture of Bacillus macerans in chemically defined medium. J. appl. Chem. Biotechnol. 23, 309–321 (1973)
Linder, D., Kurz, G., Bender, H., Wallenfels, K.: 1,4-α-glucan phosphorylase from K. pneumoniae. Purification and structure. Europ. J. Biochem. 70, 291–303 (1976)
Monod, J., Torriani, A. M.: Synthesis of a starch-type polysacharide from maltose by an enzymic preparation from bacteria. C. R. Acad. Sci. (Paris) 227, 240–242 (1948)
Nakamura, N., Horikoshi, K.: Characterization and some cultural conditions of a cyclodextrin glycosyltransferase producing alkalophilic Bacillus sp. Agr. Biol. Chem. 40 753–757 (1976)
Nelson N.: A photometric adaption of the Somogyi method for the determination of glucose. J. biol. Chem. 153 375–380 (1944)
Norrman, J., Wöber, G.: Comparative biochemistry of α-glucan utilization in Pseudomonas amyloderamosa and Pseudomonas saccharophila. Physiological significance of variations in the pathways. Arch. Microbiol. 102, 253–260 (1975)
Palmer, T. N., Ryman, B. E., Whelan, W. J.: The action of amylomaltase. FEBS Lett. 1, 1–3 (1968)
Palmer, T. N., Wöber, G., Whelan, W. J.: The pathway of exogenous and endogenous carbohydrate utilization in Escherichia coli: A dual function for the enzymes of the maltose operon. Europ. J. Biochem 39 601–612 (1973)
Robyt, J., French, D.: Purification and action pattern of an amylase from Bacillus polymyxa. Arch. Biochem. Biophys. 104, 338–345 (1964)
Sadoff, H. L.: Glucose dehydrogenase-soluble. In: Methods in enzymology Vol. IX (S. P. Colowick, N. O. Kaplan, eds.), pp. 103–107, New York-London: Academic Press 1966
Schwartz, M., Hofnung, M.: La maltodextrin phosphorylase d'Escherichia coli. Europ. J. Biochem. 2, 131–145 (1967)
Schwinn, H., Schulz, G. V.: Untersuchungen über Amylomaltase. III. Kinetische Analyse des Reaktionsmechanismus. Biochim. biophys. Acta (Amst.) 227, 313–326 (1971)
Spiro, R. G.: Analysis of sugars found in glycoproteins. In: Methods in enzymology, Vol. VIII (E. F. Neufeld, V. Ginsburg, eds.), pp. 3–36, New York-London: Academic Press 1966
Thanner, F., Palm, D., Shaltiel, S.: Hydrophobic and biospecific chromatography in the purification of maltodextrin phosphorylase from Escherichia coli. FEBS Lett. 55, 178–182 (1975)
Thoma, J. A., Stewart, L.: Cycloamyloses. In: Starch: Chemistry and technology, Vol. I, Fundamental aspects (R. L. Whistler, E. F. Paschall, eds.), pp. 209–249. New York-London: Academic Press 1965
Tilden, E. B., Hudson, C. S.: Preparation and properties of the amylases produced by Bacillus macerans and Bacillus polymyxa. J. Bact. 43 527–544 (1942)
Viles, F. J., Silverman, L.: Determination of starch and cellulose with anthrone. Analyt. Chem. 21, 950–953 (1949)
Wiesmeyer, H.: Amylomaltase. In: Methods in enzymology, Vol. V (S. P. Colowick, N. O. Kaplan, eds.), pp. 141–144, New York-London: Academic Press 1962
Wöber, G.: The pathway of maltodextrin metabolism in Pseudomonas stutzeri. Hoppe-Seylers Z. physiol. Chem. 354, 75–82 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bender, H. Cyclodextrin-Glucanotransferase von Klebsiella pneumoniae . Arch. Microbiol. 113, 49–56 (1977). https://doi.org/10.1007/BF00428579
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00428579