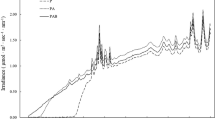
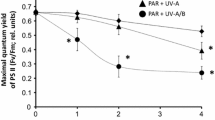
Abstract
Cnidarians which contain symbiotic algae are constantly faced with the challenges of a changing photic regime and a hyperoxic environment. Zooxanthellae (Symbiodinium sp.) from the sea anemone Aiptasia pallida (Verrill), collected and cultured at Bermuda Biological Station in 1986, exhibit a suite of compensatory responses to changes in irradiance, ultraviolet radiation (UV), and to the toxicity resulting from their interaction with photosynthetically produced oxygen. Superoxide dismutase (SOD) and catalase inactivate superoxide radicals (O2 -) and hydrogen peroxide (H2O2), which are mediators of oxygen toxicity, show an increase in specific activity with irradiance and in response to UV, both in cultured zooxanthellae (CZ) and freshly isolated zooxanthellae (FIZ) from acclimated anemones. CZ and FIZ exposed to environmentally realistic UV levels show a 30 to 40% increase in SOD activities compared with zooxanthellae exposed to similar irradiances without UV. CZ consistently show higher activities of both SOD and catalase compared to FIZ. Both CZ and FIZ exhibit changes in chlorophyll content and in the relationship between photosynthesis and irradiance which suggest photoadaptive changes in CO2-fixing enzymes, the photosynthetic-electron transport system, or in photosynthetic unit size (PSU). UV has a greater effect on the photosynthetic capacity (P max) of FIZ when compared to CZ acclimated at an equivalent irradiance with or without a UV component. UV also enhances the photoinhibition observed at high irradiance in both CZ and FIZ. Differences in enzyme activity between CZ and FIZ suggest an important role for the host in the protection of zooxanthellae against the direct effects of environmentally realistic UV while the photosynthetic performance of zooxanthellae in situ may not be as well protected.
Similar content being viewed by others
Literature cited
Ahles, M. D. (1967). Some aspects of the morphology and physiology of Symbiodinium microadriaticum. Ph. D. thesis, Fordham University, New York
Allen, J. F. (1977). Superoxide and photosynthetic reduction of oxygen. In: Michelson, A. M., McCord, J. M., Fridovich, I. (eds.). Superoxide and superoxide dismutases. Academic Press, New York, p. 417–436
Asada, K., Kanematsu, S., Okada, S., Hayakawa, T. (1980). Phylogenetic distribution of three types of superoxide dismutase in organisms and in cell organelles. In: Bannister, J. V., Hill, H. A. O. (eds.). Chemical and biochemical aspects of superoxide and superoxide dismutase. Elsevier, Amsterdam, p. 136–153
Asada, K., Takahashi, M. (1987). Production and scavenging of active oxygen in photosynthesis. In: Kyle, D. J., Osmond, C. B., Arntzen, C. J. (eds.). Photoinhibition. Elsevier, Amsterdam, p. 228–287
Bannister, J. V., Bannister, W. H., Rotilio, G. (1987). Aspects of the structure, function, and applications of superoxide dismutase. In: CRC Critical Reviews in Biochemistry. Boca Raton, FL., Vol. 22, Issue 2, p. 111–180
Beauchamp, C., Fridovich, I. (1971). Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Analyt. Biochem. 44: 276–285
Beers, R. F., Jr., Sizer, I. W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. biol. Chem. 195: 133–140
Berland, B. R., Maestrini, S. Y. (1969). Study of bacteria associated with marine algae in culture. II. Action of antibiotic substances. Mar. Biol. 3: 334–335
Berner, T., Achituv, Y., Dubinsky, Z., Benayahu, Y. (1987). Pattern of distribution and adaptation to different irradiance levels of zooxanthellae in the soft coral Litophyton arboreum (Octocorallia, Alcyonacea). Symbiosis 3: 23–40
Burns, B. D., Beardall, J. (1987). Utilization of inorganic carbon by marine microalgae. J. exp. mar. Biol. Ecol. 107: 75–86
Calkins, J., Thordardottir, T. (1980). The ecological significance of solar UV radiation on aquatic organisms. Nature, Lond. 283: 563–566
Cavanaugh, G. M. (1975). Formulae and methods VI of the Marine Biological Laboratory Chemical Room, Marine Biological Laboratory, Woods Hole, MA
Chang, S. S., Prézelin, B. B., Trench, R. K. (1983). Mechanisms of photoadaptation in three strains of the symbiotic dinoflagellate Symbiodinium microadriaticum. Mar. Biol. 76: 219–229
Claustre, H., Gostan, J. (1987). Adaptation of biochemical composition and cell size to irradiance in two microalgae: possible ecological implications. Mar. Ecol. Prog. Ser. 40: 167–174
Clayton, R. K. (1977). Light and living matter, Vol. 2. The biological part. Robert E. Krieger Publ. Co., Huntington, New York
Coles, S. L., Jokiel, P. L. (1978). Synergistic effects of temperature, salinity and light on the hermatypic coral Montipora verrucosa. Mar. Biol. 49: 187–195
Cook, C. B. (1983). Metabolic interchange in algae-invertebrate symbiosis. Int. Rev. Cytol. Suppl. 14: 177–209
Crossland, C. J., Barnes, D. J. (1977). Gas exchange studies with the staghorn coral Acropora acuminata and its zooxanthellae. Mar. Biol. 40: 185–194
Davis, B. J. (1964). Disc electrophoresis — II method and application to human serum proteins. Ann. N. Y. Acad. Sci. 121: 404–427
D'Aoust, B. G., White, R., Wells, J. M., Olsen, D. A. (1976). Coralalgal associations: capacity for producing and sustaining elevated oxygen tensions in situ. Undersea Biomed. Res. 3: 35–40
Dunlap, W. C., Chalker, B. E., Oliver, J. K. (1986). Bathymetric adaptations of reef-building corals at Davies Reef, Great Barrier Reef, Australia. III. UV-B absorbing compounds. J. exp. mar. Biol. Ecol. 104: 239–248
Dustan, P. (1982). Depth-dependent photoadaptation by zooxanthellae of the reef coral Montastrea annularis. Mar. Biol. 68: 253–264
Dykens, J. A. (1984). Enzymic defenses against oxygen toxicity in marine cnidarians containing endosymbiotic algae. Mar. Biol. Lett. 5: 291–301
Dykens, J. A., Shick, J. M. (1982). Oxygen production by endosymbiotic algae controls superoxide dismutase activity in their animal host. Nature, Lond. 297: 579–580
Dykens, J. A., Shick, J. M. (1984). Photobiology of the symbiotic sea anemone, Anthopleura elegantissima: defenses against photodynamic effects, and seasonal photoacclimatization. Biol. Bull. mar. biol Lab., Woods Hole 167: 683–697
Elstner, E. F. (1982). Oxygen activation and oxygen toxicity. A. Rev. Pl. Physiol. 33: 73–96
Elstner, E. F., Heupel, A. (1976). Inhibition of nitrite formation from hydroxylammonium-chloride: a simple assay for super-oxide dismutase. Analyt. Biochem. 70: 616–620
Falkowski, P. G., Dubinsky, Z., Santostefano, G. (1985). Light-enhanced dark respiration in phytoplankton. Verh. Internat. Verein. Limnol. 22: 2830–2833
Foote, C. S. (1976). Photosensitized oxidation and singlet oxygen: consequences in biological systems. In: Pryor, W. A. (ed.). Free radicals in biology. Academic Press, New York, Vol. II, p. 85–133
Freeman, B. A., Crapo, J. D. (1982). Biology of disease: free radicals and tissue injury. Lab. Invest. 47: 412–426
Fridovich, I. (1978) The biology of oxygen radicals. Science, N.Y. 201: 875–880
Fridovich, I. (1981). Superoxide radical and superoxide dismutases. In: Schaefer, K. E., Gilbert, D. L. (eds.) Oxygen and living processes. Springer-Verlag, New York, p. 250–272
Fridovich, I. (1986). Biological effects of the superoxide radical. Archs Biochem. Biophys. 247: 1–11
Gallagher, J. C., Alberte, R. S. (1985). Photosynthetic and cellular photoadaptive characteristics of three ecotypes of the marine diatom, Skeletonema costatum (Grev.) Cleve. J. exp. mar. Biol. Ecol. 94: 233–250
Gallagher J. C., Wood, A. M., Alberte, A. M. (1984). Ecotypic differentiation in the marine diatom Skletonema costatum: influence of light intensity on the photosynthetic apparatus. Mar. Biol. 82: 121–134
Glynn, P. W., Peters, E. S., Muscatine, L. (1985). Coral tissue microstructure and necrosis: relation to catastrophic coral mortality in Panamá. Dis. aquat. Organisms 1: 29–37
Goreau, T. F. (1964). Mass expulsion of zooxanthellae from Jamaican reef communities after hurricane Flora. Science, N.Y. 145: 383–386
Groden, D., Beck, E. (1979). H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim. Biophys. Acta. 546: 426–435
Guillard, R. R. L. (1973). Division rates. In: Stein, J. R. (ed.). Handbook of phycological methods-culture methods and growth measurements. Cambridge University Press, Boston, p. 290–311
Halldal, P. (1968). Photosynthetic capacities and photosynthetic action spectra of endozoic algae of the massive coral Favia. Biol. Bull. mar. biol. Lab., Woods Hole 134: 411–424
Halliwell, B. (1982). The toxic effects of oxygen on plant tissues. In: L. W. Oberley (ed.). Superoxide dismutase. CRC Press, Boca Raton, Vol. I., p. 89–123
Hill, H. A. O. (1981). Oxygen, oxidases, and the essential trace metals. Phil. Trans. R. Soc. Lond. (Ser B) 194: 119–128
Imlay, J. A., Chin, S. M., Linn, S. (1988). Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science, N.Y. 240: 640–642
Itzhaki, R. F., Gill, D. M., (1964). A micro-biuret method for estimating proteins. Analyt. Biochem. 9: 401–410
Jaap, W. C. (1979). Observations on zooxanthellae expulsion at Middle Sambo Reef, Florida Keys. Bull. mar. Sci. 29: 414–422
Jeffrey, S. W., Humphrey, G. F. (1975). New spectrophotometric equations for determing chlorophylls a, b, c, and c 2 in higher plants, algae and natural phytoplankton. Biochem. Biophys. Pfl. 167: 191–194
Jokiel, P. L. (1980). Solar ultraviolet radiation and coral reef epifauna. Science, N.Y. 207: 1069–1071
Jokiel, P. L., York, R. H. Jr. (1982). Solar ultraviolet photobiology of the reef coral Pocillopora damicornis and symbiotic zooxanthellae. Bull. mar. Sci. 32: 301–315
Jokiel, P. L., York, R. H. Jr. (1984). Importance of ultraviolet radiation in photoinhibition of microalgal growth. Limnol. Oceanogr. 29: 192–199
Krause, G. H., Cornic, G. (1987). CO2 and O2 interactions in photoinhibition. In: Kyle, D. J., Osmond, C. B., Arntzen, C. J. (eds.). Photoinhibition. Elsevier, Amsterdam, p. 169–196
Kyle, D. J. (1987). The biochemical basis for photoinhibition of photosystem II. In: Kyle, D. J., Osmond, C. B., Arntzen, C. J. (eds.). Photoinhibition. Elsevier, Amsterdam, p. 197–226
Kyle, D. J., Ohad, I. (1986). The mechanism of photoinhibition in higher plants and green algae. In: Staehlin, L. A., Arntzen, C. J. (eds.). Encyclopedia of plant physiology, Vol. 19. Photosynthesis III, photosynthetic membranes and light harvesting systems. Springer-Verlag, Berlin, p. 468–475
Lewis, M. R., Smith, J. C. (1983). A small volume, short-incubation-time method for measuring photosynthesis as a function of incident irradiance. Mar. Ecol. Prog. Ser. 13: 99–102
Ludlow, M. M. (1987). Light stress at high temperature. In: Kyle, D. J., Osmond, C. B., Arntzen, C. J. (eds.). Photoinhibition. Elsevier, Amsterdam, p. 89–110
Margulis, L. (1981). Symbiosis in cell evolution. W.H. Freeman and Company, San Francisco
Maske, H. (1984). Daylight ultraviolet radiation and the photoinhibition of phytoplankton carbon uptake. J. Plankton Res. 6: 351–357
McAuley, P. J. (1986). Isolation of viable uncontaminated Chlorella from green hydra. Limnol. Oceanor. 31: 222–224
Muller-Parker, G. (1984). Photosynthesis-irradiance responses and photosynthetic periodicity in the sea anemone Aiptasia pulchella and its zooxanthellae. Mar. Biol. 82: 225–232
Muller-Parker, G. (1987). Seasonal variation in light-shade adaptation of natural populations of the symbiotic sea anemone Aiptasia pulchella (Carlgren, 1943) in Hawaii. J. exp. mar. Biol. Ecol. 112: 165–183
Muscatine, L., McCloskey, L. R., Martin, R. E. (1981). Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 26: 601–611
Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Pl. Cell Physiol., Tokyo 22: 867–880
Neale, P. J. (1987). Algal photoinhibition and photosynthesis in the aquatic environment. In: Kyle, D. J., Osmond, C. B., Arntzen, C. J. (eds.). Photoinhibition. Elsevier, Amsterdam, p. 39–65
Ogren, W. L. (1984). Photorespiration: pathways, regulation, and modification. A. Rev. Pl. Physiol. 35: 415–442
Osmond, C. B. (1981). Photorespiration and photoinhibition, some implications for the energetics of photosynthesis. Biochim. Biophys. Acta. 639: 77–98
Oyanagui, Y. (1984). Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Analyt. Biochem. 142: 290–296
Palincsar, J. S., Jones, W. R., Palincsar, E. E. (1988). Effects of isolation of the endosymbiont Symbiodinium microadriaticum (Dinophyceae) from its host Aioptasia pallida (Anthozoa) on cell wall ultrastructure and mitotic rate. Trans. Am. microsc. Soc. 107: 53–66
Patton, J. S., Burris, J. E. (1983). Lipid droplets, medium of energy exchange in the symbiotic anemone Condylactis gigantea: a model coral polyp. Mar. Biol. 75: 137–149
Platt, T., Gallegos, C. L., Harrison, W. G. (1980). Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. mar. Res. 38: 687–701
Porter, J. W., Muscatine, L., Dubinsky, Z., Falkowski, P. G. (1984). Primary production and photoadaptation in light- and shadeadapted colonies of the symbiotic coral, Stylophora pistillata. Proc. R. Soc. Lond. (Ser. B) 222: 161–180
Powles, S. B. (1984). Photoinhibition of photosynthesis induced by visible light. A. Rev. Pl. Physiol. 35: 15–44
Prézelin, B. B. (1987). Photosynthetic physiology of dinoflagellates. In: Taylor, F. J. R. (ed.). The biology of dinoflagellates. Blackwell, Oxford, p. 174–223
Ralston, M. L., Jenrich, R. I. (1978). DUD, a derivative-free algorithm for nonlinear least squares. Technometrics 20: 7–14
Renger, G., Voss, M., Gräber, P., Schulze, A. (1986). Effects of UV irradiation on different partial reactions of the primary processes of photosynthesis. In: Worrest, R. C., Caldwell, M. M. (eds.) Stratispheric ozone reduction, solar ultraviolet radiation and plant life. Springer-Verlag, New York, p. 171–184
Richardson, K., Beardall, J., Raven, J. A. (1983). Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytol. 93: 157–191
Roberts, L. (1987). Coral bleaching threatens Atlantic reefs. Science, N.Y. 238: 1228–1229
SAS Institute (1982). SAS user's guide: statistics SAS Institute Inc., Cary, North Carolina, USA
Schoneberg, D. A., Trench, R. K. (1980). Genetic variation in Symbiodinium microadriaticum (Freudenthal) and specificity in its symbiosis with marine invertebrates. II. Morphological variation in S. microadriaticum. Proc. R. Soc. Lond. (Ser. B) 207: 429–444
Shick, J. M., Dykens, J. A. (1985). Oxygen detoxification in algal-invertebrate symbioses from the Grerat Barrier Reef. Oecologia 66: 33–41
Siebeck, O. (1988). Experimental investigation of UV tolerance in hermatypic corals (Scleractinia). Mar. Ecol. Prog. Ser. 43: 95–103
Sisson, W. B. (1986). Effects of UV-B radiation on photosynthesis. In: Worrest, R. C., Caldwell, M. M. (eds.) Stratispheric ozone reduction, solar ultraviolet radiation and plant life. Springer-Verlag, New York, p. 161–169
Smith, C. S., Baker, K. S. (1979). Penetration of UV-B and biologically effective dose-rates in natural waters. Photochem. Photobiol. 29: 311–323
Sokal, R. R., Rohlf, F. J. (1979). Biometry, 2nd ed. W. H. Freeman and Co., San Francisco
Spinrad, R. W., Yentsch, C. M. (1987). Observations on the intra-and interspecific single cell optical variability of marine phytoplankton. Appl. Optics (Easton, Pa.) 26: 357–362
Steen, R. G. (1986). Evidence for heterotrophy by zooxanthellae in symbiosis with Aiptasia pulchella. Biol. Bull. mar. biol. Lab, Woods Hole. 170: 267–278
Sterrer, W. (ed.) (1986). Marine flora and fauna of Bermuda. Wiley-Interscience, New York
Thinh, L. V., Griffiths, D. J., Winsor, H. (1986). Ultrastructure of Symbiodinium microadriaticum (Dinophyceae) symbiotic with Zoanthus sp. (Zoanthidea). Phycologia 25: 178–184
Trench, R. K. (1979). The cell biology of plant-animal symbiosis. A. Rev. Pl. Physiol. 30: 485–531
Trench, R. K., Blank, R. J. (1987). Symbiodinum microadriaticum Freudenthal, S. Goreauii sp. nov., S. kawagutii sp. nov., S. pilosum sp. nov.: gymnodinioid dinoflagellate symbionts of marine invertebrates. J. Phycol., 23: 469–481
Tytler, E. M., Trench, R. K. (1986). Activities of enzymes in β-carboxylation reactions and of catalase in cell-free preparations from the symbiotic dinoflagellates Symbiodinium spp. from a coral, a clam, a zoanthid and two sea anemones. Proc. R. Soc. Lond. (Ser. B) 228: 483–492
Valenzeno, D. P., Pooler, J. P. (1987). Photodynamic action. BioSci. 37: 270–276
Weis, V. M., Smith, G. J., Muscatine, L. (1989). A “CO2 supply” mechanism in zooxanthellate cnidarians: role of carbonic anhydrase. Mar. Biol. 100: 195–202
Wilkerson, F. P., Kobayashi, D., Muscatine, L. (1988). Mitotic index and size of symbiotic algae in Caribbean reef corals. Coral Reefs 7: 29–36
Wood, W. F. (1987). Effect of solar ultra-violet radiation on the kelp Ecklonia radiata. Mar. Biol. 96: 143–150
Worrest, R. C. (1982). Review of literature concerning the impact of UV-B radiation upon marine organisms In: Calkins, J. (ed.). The role of solar ultraviolet radiation in marine ecosystems. Plenum Press, New York, p. 429–457
Yentsch, C. M., Horan, P. K., Muirhead, K., Dortch, Q., Haugen, E. Legendre, L., Murphy, L. S., Perry, M. J., Phinney, D. A., Pomponi, S. A., Spinrad, R. W., Wood, M., Yentsch, C. S., Zahuranec, B. J. (1983). Flow cytometry and cell sorting: a technique for analysis and sorting of aquatic particles. Limnol. Oceanogr. 28: 1275–1280
Zar, J. H. (1984). Biostatistical analysis. Prentice-Hall, Inc., Englewood Cliffs, N. J.
Zimmerman, R. C., SooHoo, J. B., Kremer, J. N., D'Argenio, D. Z. (1987). Evaluation of variance approximation techniques for non-linear photosynthesis-irradiance models. Mar. Biol. 95: 209–215
Zvalinskii, V. I., Leletkin, V. A., Titlyanov, E. A., Shaposhnikova, M. G. (1980). Photosynthesis and adaptation of corals to irradiance. 2. Oxygen exchange. Photosynthetica (Praha, Czecholslovakia) 14: 422–430
Author information
Authors and Affiliations
Additional information
Communicated by J. Grassle, Woods Hole
Rights and permissions
About this article
Cite this article
Lesser, M.P., Shick, J.M. Effects of irradiance and ultraviolet radiation on photoadaptation in the zooxanthellae of Aiptasia pallida: primary production, photoinhibition, and enzymic defenses against oxygen toxicity. Mar. Biol. 102, 243–255 (1989). https://doi.org/10.1007/BF00428286
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00428286