Abstract
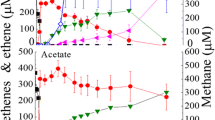
Methanogenic enrichment cultures with isobutyrate as sole source of carbon and energy were inoculated with sediment and sludge samples from freshwater and marine origin. Over more than 20 transfers, these cultures fermented 2 mol isobutyrate with 1 mol CO2 via an intermediate formation of n-butyrate to 4 mol acetate and 1 mol CH4. The primary isobutyrate-fermenting bacteria could not be purified. From one of the marine enrichment cultures, a sulfate-reducing bacterium was isolated which oxidized isobutyrate with sulfate completely to CO2. Based on its physiological and morphological properties, this strain was assigned to the known species Desulfococcus multivorans. It also oxidized many other fatty acids without significant release of short-chain intermedeates. The enzymes involved in isobutyrate degradation by this bacterium were assayed in cell-free extracts. The results indicate that isobutyrate is activated to its CoA derivative and oxidized via methylmalonate semialdehyde to propionyl-CoA. Propionyl-CoA is further converted via the methylmalonyl-CoA pathway to acetyl-CoA which is finally cleaved by the CO-dehydrogenase system. It is evident that this is not the pathway used by the fermenting bacteria prevailing in the methanogenic enrichment cultures. There results are discussed on the basis of energetical considerations.
Similar content being viewed by others
References
Allison MJ (1969) Biosynthesis of amino acids by ruminal microorganisms. J Anim Sci 29:797–807
Allison MJ (1978) Production of branched-chain volatile fatty acids by certain anaerobic bacteria. Appl Environ Microbiol 35: 872–877
Allison MJ, Bryant MP (1963) Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria. Arch Biochem Biophys 101:262–277
Barker HA (1981) Amino acid degradation by anaerobic bacteria. Ann Rev Biochem 50:23–40
Bergmeyer HU (ed) (1974) Methoden der enzymatischen Analyse, 3rd edn. Verlag Chemie, Weinheim
Bernt E, Bergmeyer HU (1974) Isocitrate dehydrogenase. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, vol 1. Verlag Chemie, Weinheim, pp 664–667
Brandis-Heep A, Gebhardt NA, Thauer RK, Widdel F, Pfennig N (1983) Anaerobic acetate oxidation to CO2 by Desulfobacter postgatei. 1. Demonstration of all enzymes required for the operation of the citric acid cycle. Arch Microbiol 136:222–229
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Green DE, Mii S, Mahler HR, Bock RM (1954) Studies on the fatty acid oxidizing system of animal tissue. III. Butyryl coenzyme A dehydrogenase. J Biol Chem 206:1–12
Gregersen T (1978) Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur J Appl Microbiol Biotechnol 5:123–127
Gustafson WG, Feinberg BA, McFarland JT (1986) Energetics of β-oxidation. Reduction potentials of general fatty acyl-CoA dehydrogenase, electron transfer flavoprotein, and fatty acyl-CoA substrates. J Biol Chem 261:7733–7741
Harwood CS, Canale-Parola E (1981) Branched-chain amino acid fermentation by a marine spirochete: strategy for starvation survival. J Bacteriol 148:109–116
Hilpert W, Schink B, Dimroth P (1984) Life by a new decarboxylation-dependent energy conservation mechanism with Na+ as coupling ion. EMBO J 3:1665–1670
Knappe J, Blaschkowski HP, Gröbner P, Schmit T (1974) Pyruvate formate lyase of Escherichia coli: the acetyl-enzyme intermediate. Eur J Biochem 50:253–263
Koch M, Dolfing J, Wuhrmann K, Zehnder AJB (1983) Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol 45:1411–1414
Kuenen JG, Veldkamp H (1972) Thiomicrospira pelophila gen. nov. sp. n., a new obligately chemolithotrophic colourless sulfur bacterium. Antonie van Leeuwenhoek J Microbiol Serol 38:241–256
Lee MJ, Zinder SH (1988) Isolation and characterization of a thermophilic bacterium which oxidizes acetate in syntrophic association with a methanogen and which grows acetogenically on H2−CO2. Appl Environ Microbiol 54:124–129
Lovley DR, Klug MJ (1982) Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol 43:552–560
Magee CM, Rodeheaver G, Edgerton MT, Edlich RF (1975) A more reliable Gram staining technic for diagnosis of surgical infections. Am J Surgery 130:341–346
Massey LK, Sokatch JR, Conrad RS (1976) Branched-chain amino acid catabolism in bacteria. Bacteriol Rev 40:42–54
Moskowitz GJ, Merrick JM (1969) Metabolism of poly-β-hydroxybutyrate. II. Enzymatic synthesis of d-(-)β-hydroxybutyryl coenzyme A by an enoyl hydrase from Rhodospirillum rubrum. Biochemistry 8:2748–2754
Pfennig N (1978) Rhodocyclus purpureus gen. nov. and sp. nov., a ring-shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28:283–288
Rendina G, Coon MJ (1957) Enzymatic hydrolysis of the coenzyme A thiol esters of β-hydroxypropionic and β-hydroxyisobutyric acids. J Biol Chem 225:523–534
Robinson WG, Coon MJ (1957) The purification and properties of β-hydroxyisobutyric dehydrogenase. J Biol Chem 225:511–521
Robinson WG, Nagle R, Bachhawat BK, Kupiecki FP, Coon MJ (1957) Coenzyme A thiol esters of isobutyric, methacrylic, and β-hydroxyisobutyric acids as intermediates in the enzymatic degradation of valine. J Biol Chem 224:1–11
Sansone FJ, Martens CS (1981) Determination of volatile fatty acid turnover rates in organic-rich marine sediments. Mar Chem 10:233–247
Sansone FJ, Martens CS (1982) Volatile fatty acid cycling in organicrich marine sediments. Geochim Cosmochim Acta 46:1575–1589
Schauder R, Eikmanns B, Thauer RK, Widdel F, Fuchs G (1986) Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch Microbiol 145:162–172
Schink B (1985a) Fermentation of acetylene by an obligate anaerobe, Pelobacter acetylenicus sp. nov. Arch Microbiol 142:295–301
Schink B (1985b) Mechanism and kinetics of succinate and propionate degradation in anoxic freshwater sediments and sewage sludge. J Gen Microbiol 131:643–650
Schink B, Pfennig N (1982) Fermentation of trihydroxybenzenes by Pelobacter acidigallici gen. nov. sp. nov., a new strictly anaerobic, non-sporeforming bacterium. Arch Microbiol 133:195–201
Schink B, Thauer RK (1988) Energetics of syntrophic methane formation and the influence of aggregation. In: Lettinga G, Zehnder AJB, Grotenhuis JTC, Hulshoff Pol LW (eds) Granular anaerobic sludge; microbiology and technology. Pudoc, Wageningen, pp 5–17
Schink B, Kremer DR, Hansen ThA (1987) Pathway of proprionate formation from ethanol in Pelobacter propionicus. Arch Microbiol 147:321–327
Scrutton MC, Olmsted MR, Utter MF (1969) Pyruvate carboxylase from chicken liver. Meth Enzymol 13:235–249
Shelton DR, Tiedje JM (1984) Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic acid. Appl Environ Microbiol 48:840–848
Sokatch JR (1966) Alanine and aspartate formation during grwoth on valine-14C by Pseudomonas aeruginosa. J Bacteriol 92:72–81
Sokatch JR, Sanders LE, Marshall VP (1968) Oxidation of methylmalonate semialdehyde to propionyl coenzyme A in Pseudomonas aeruginosa grown on valine. J Biol Chem 243:2500–2506
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA (1984) Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Stieb M, Schink B (1984) A new 3-hydroxybutyrate-fermenting anaerobe, Ilyobacter polytropus gen. nov. sp. nov., possessing various fermentation pathways. Arch Microbiol 140:139–146
Stieb M, Schink B (1985) Anaerobic oxidation of fatty acids by Clostridium bryantii so. nov., a sporeforming, obligately syntrophic bacterium. Arch Microbiol 140:387–390
Stieb M, Schink B (1986) Anaerobic degradation of isovalerate by a defined methanogenic coculture. Arch Microbiol 144:291–295
Tanner RS, Wolfe RS (1988) Nutritional requirements of Methanomicrobium mobile. Appl Environ Microbiol 54:625–628
Thauer RK, Morris JG (1984) Metabolism of chemotrophic anaerobes: old views and new aspects. In: Kelly DP, Carr NG (eds) The microbe 1984 Part II. Prokaryotes and eukaryotes. Soc Gen Microbiol Symp, vol 36. Cambridge University Press, Cambridge, pp 123–168
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Tholozan J-L, Samain E, Grivet J-P (1988) Isomerization between n-butyrate and isobutyrate in enrichment cultures. FEMS Microbiol Ecol 53:187–191
Widdel F (1988) Microbiology and ecology of sulfate- and sulfurreducing bacteria. In: Zehnder AJB (ed) Environmental biology of anaerobes. John Wiley, New York (in press)
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov. sp. nov. Arch Microbiol 129:395–400
Widdel F, Pfennig N (1984) Dissimilatory sulfate- or sulfuroxidizing bacteria. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore London, pp 663–679
Widdel F, Kohring G-W, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134:286–294
Wofford NQ, Beaty PS, McInerney MJ (1986) Preparation of cellfree extracts and the enzymes involved in fatty acid metabolism in Syntrophomonas wolfei. J Bacteriol 167:179–185
Zinder SH, Koch M (1984) Non-aceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic syntrophic coculture. Arch Microbiol 138:263–272
Zinder SH, Cardwell SC, Anguish T, Lee M, Koch M (1984) Methanogenesis in a thermophilic (58°C) anaerobic digestor: Methanothrix sp. as an important aceticlastic methanogen. Appl Environ Microbiol 47:796–807
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stieb, M., Schink, B. Anaerobic degradation of isobutyrate by methanogenic enrichment cultures and by a Desulfococcus multivorans strain. Arch. Microbiol. 151, 126–132 (1989). https://doi.org/10.1007/BF00414426
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00414426