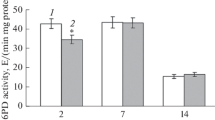
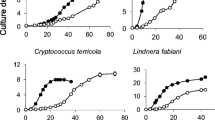
Abstract
Compartmentation of the metabolism of ethylamine in Trichosporon cutaneum X4 was studied in cells, grown on this compound as the sole source of energy, carbon, and nitrogen. Transfer experiments indicated that an amine oxidase is involved in the early metabolism of ethylamine. The synthesis of this enzyme was induced by primary amines and was subject to partial carbon catabolite repression. Repression by ammonium ions was not observed. Adaptation of glucose-grown cells to growth on ethylamine was associated with the development of many microbodies, which developed from already existing organelles present in the inoculum cells and multiplied by division. Cytochemical experiments indicated that the organelles contained amine oxidase and catalase. Therefore, they were considered to play a key role in the metabolism of ethylamine. The physiological significance of the microbodies was investigated by fractionation studies of homogenized protoplasts from ethylamine-grown cells by differential- and sucrose-gradient centrifugation of subcellular organelles. Intact microbodies were only obtained when the isolation procedure was performed at pH 5.8 in the absence of Mg2+-ions. Analysis of the different fractions indicated that the key enzymes of the glyoxylate cycle, namely isocitrate lyase and malate synthase, cosedimented together with catalase and amine oxidase. In addition, activities of malate dehydrogenase, glutamate:oxaloacetate aminotransferase (GOT) and (NAD-dependent) glutamate dehydrogenase were detected in these fractions. Electron microscopy revealed that they mainly contained microbodies. Cytochemical experiments indicated that the above enzymes were all present in the same organelle. These findings suggest that microbodies of ethylamine-grown T. cutaneum X4 produce aspartate, so allowing NADH generated in the oxidation of malate by malate dehydrogenase to be quantitatively reoxidized inside the organelles in a series of reactions involving GOT and glutamate dehydrogenase. Aspartase and fumarase were not detected in the microbodies; activities of these two enzymes were present in the cytoplasm.
Similar content being viewed by others
Abbreviations
- ABTS:
-
2,2′-Azino-di(3-ethylbenzthiazoline sulfonate [6])
- DTT:
-
dithiothreitol
- GOT:
-
glutamate:oxaloacetate aminotransferase
- DTNB:
-
5,5-dithiobis-2-nitrobenzoate
- DAB:
-
diaminobenzidine
- BSPT:
-
2-(2-benzothiazolyl)-3-(4-phthalhydrazidyl)-t-styryl-sH-tetrazolium chloride
- PF:
-
convex fracture face
- EF:
-
concave fracture face
References
Altman FP (1976) Tetrazolium salts and formazans. Progr Histochem Cytochem, vol 9, no 3. Fischer, Stuttgart New York
Bergmeyer HU, Bernt E (1974a) GOT. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York San Francisco London, pp 727–733
Bergmeyer HU, Bernt E (1974b) Malate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York San Francisco London, pp 613–617
Bergmeyer HU, Gawehn K, Grassl M (1974c) Enzymes as Biochemical Reagents, Uricase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York San Francisco London, pp 518
Bergmeyer HU, Gawehn K, Grassl M (1974d) Enzymes as Biochemical Reagents, Acid Phosphatase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York San Francisco London, pp 495–496
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Branton D, Bullivant S, Gilula NB, Karnovsky MJ, Moor H, Mühlethaler K, Northcote DH, Packer L, Satir B, Satir P, Speth V, Staehelin LA, Steere RL, Weinstein RS (1975) Freeze etching nomenclature. Science 190:54–55
Dijken JP van, Bos P (1981) Utilization of amines by yeasts. Arch Microbiol 128:320–324
Dijken JP van, Veenhuis M, Vermeulen CA, Harder W (1975) Cytochemical localization of catalase activity in methanol-grown Hansenula polymorpha. Arch Microbiol 105:216–267
Dixon G, Kornberg HL (1959) Assay methods for key enzymes of the glyoxylate cycle. Biochem J 72:3–9
Duve C De, Baudhuin P (1966) Peroxisomes (microbodies and related particles). Physiol Rev 46:323–357
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspension. Nature Phys Sci 241:20–22
Goodman JM, Scott CW, Donahue PN, Atherton JP (1984) Alcohol oxidase assembles posttranslationally into the peroxisome of Candida boidinii. J Biol Chem 259:8485–8493
Harder W, Visser K, Kuenen JG (1974) Laboratory fermenter with an improved magnetic drive. Lab Practice 23:644–645
Haywood GW, Large PJ (1981) Microbial oxidation of amines. Distribution, purification and properties of two primary amine oxidases from the yeast Candida boidinii grown on amines as sole nitrogen source. Biochem J 199:187–201
Hill RL, Bradshaw RA (1968) Fumarase. In: Lowenstein (ed) Methods in Enzymology, vol XIII. Academic Press, New York London, pp 91–92
Kun E, Kearney EB (1974) Ammonia. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York San Francisco London, pp 1802–1806
Lee SH, Torack RM (1968) Electron microscope studies of glutamic oxalacetic transaminase in rat liver cell. J Cell Biol 39:716–724
Lück H (1963) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, London New York, pp 885–894
Mandels M, Reese ET (1957) Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol 73:269–278
Middelhoven WJ, Hoogkamer-te Niet MC (1984) Yeasts utilizing amines as sole source of carbon and energy. Abstr. 6th Int Symp on Yeasts, Montpellier, France II 1-P
Middelhoven WJ, Hoogkamer-te Niet MC, Kreger-van Rij NJW (1984) Trichosporon adenivorans spec. nov., a yeast species, utilizing adenine, xanthine, uric acid, putrescine and primary nalkylamines as the sole source of carbon nitrogen and energy. Antonie van Leeuwenhoek J Microbiol Serol 50:369–378
Moor H (1964) Die Gefrier-Fixation lebender Zellen und ihre Anwendung in der Elektronen-Mikroskopie. Z Zellforsch 62:546–580
Osumi M, Imaizumi F, Imai M, Sato H, Yamaguchi H (1975) Isolation and characterization of microbodies from Candida tropicalis PK 233 cells grown on normal alkanes. J Gen Appl Microbiol 21:375–387
Papadimitrion JM, van Duyn P (1970) Effects of fixation and substrate protection on the isoenzymes of aspartate aminotransferase studied in a quantitative cytochemical model system. J Cell Biol 47:71–83
Potter VR (1955) Tissue honogenates. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 1. Academic Press, New York, pp 10–15
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Scheibe P, Bernt E, Bergmeyer HU (1974) Uric acid. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York San Francisco London, pp 1951–1958
Schmidt E (1974) Glutamate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 2nd edn. Academic Press, New York San Francisco London, pp 650–656
Slot JW, Geuze HJ (1984) Gold markers for single and double immunolabeling of ultrathin cryoections. In: Polak JM, Varndell JM (eds) Immunolabeling for electron microscopy. Elsevier Sci Publ, Amsterdam, pp 129–142
Srere PA, Brazil H, Gonen L (1963) Malate synthase. Acta Chem Scand 17:129
Switzer RL (1977) The inactivation of microbial enzymes in vivo. Ann Rev Microbiol 31:135–157
Theimer RR, Wanner G, Anding G (1978) Isolation and biochemical properties of two types of microbody from Neurospora crassa cells. Cytobiology 18:132–144
Thomulka KW, Moat AG (1972) Inorganic nitrogen assimilation in yeasts: alteration in enzyme activities associated with changes in cultural conditions and growth phase. J Bacteriol 109:25–33
Tolbert NE (1981) Metabolic pathways in peroxisomes and glyoxysomes. Ann Rev Biochem 50:133–157
Trelease RN (1975) Malate synthase. In: Hayat MA (ed) Electron microscopy of enzymes vol 4. Van Nostrand Reinhold, New York Cincinnatti Toronto London Melbourne, pp 157–176
Veenhuis M, Harder W (1986) Yeast microbodies. Their substructure, biogenesis and turnover in relation to environmental conditions. In: Rose AH (ed) The yeasts, vol 3. Academic Press, London (in press)
Veenhuis M, Van Dijken JP, Harder W (1976) Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch Microbiol 111:123–125
Veenhuis M, Keizer I, Harder W (1979) Characterization of peroxisomes in glucose-grown Hansenula polymorpha and their development after the transfer of cells into methanol-containing media. Arch Microbiol 120:167–175
Veenhuis M, Van Dijken JP, Harder W (1980) A new method for the cytochemical demonstration of phosphatase activities in yeasts based on the use of cerous ions. FEMS Microbiol Lett 9:285–291
Veenhuis M, Zwart KB, Harder W (1981) Biogenesis and turnover of peroxisomes involved in the concurrent oxidation of methanol and methylamine in Hansenula polymorpha. Arch Microbiol 129:35–41
Veenhuis M, Douma A, Harder W, Osumi M (1983a) Degradation and turnover of peroxisomes in the yeast Hansenula polymorpha induced by selective inactivation of peroxisomal enzymes. Arch Microbiol 134:193–203
Veenhuis M, Dijken JP van, Harder W (1983b) The significance of peroxisomes in the metabolism of one-carbon compounds in yeasts. Adv Micr Physiol 24:1–82
Veenhuis M, Van der Klei IJ, Middelhoven WK, Harder W (1984) Isolation and characterization of yeasts capable of utilizing primary amines as the sole source of carbon and nitrogen. Abstr 6th Int Symp on Yeasts, Montpellier, France I 8-P
Veenhuis M, Hoogkamer-te Niet MC, Middelhoven WJ (1985) Biogenesis and metabolic significance of microbodies in urate-utilizing yeasts. Antonie van Leeuwenhoek J Microbiol Serol 51:31–38
Vishniac W, Santer M (1957) The thiobacilli. Bacteriol Rev 21:195–213
Vries OMH de (1974) Formation and cell wall regeneration of protoplasts from Schizophyllum commune. University of Groningen, PhD Thesis, pp 4–5
Wiemken A, Schellenberg M, Urech K (1979) Vacuoles: the sole compartments of digestive enzymes in yeasts (Saccharomyces cerevisiae). Arch Microbiol 123:23–25
Williams VR, Lartigue DJ (1969) Aspartase. In: Lowenstein JM (ed) Methods in enzymology, vol XIII, Academic Press, New York London, pp 354–355
Zwart KB (1983) Metabolic significance of microbodies in the yeasts Candida utilis and Hansenula polymorpha. PhD Thesis, University of Groningen, The Netherlands
Zwart KB, Harder W (1983) Regulation of some alkylated amines in the yeasts Candida utilis and Hansenula polymorpha. J Gen Microbiol 129:3157–3169
Zwart KB, Veenhuis M, van Dijken JP, Harder W (1980) Development of amine oxidase containing peroxisomes in yeasts during growth on glucose in the presence of methylamine as the sole nitrogen source. Arch Microbiol 126:117–126
Zwart KB, Veenhuis M, Plat G, Harder W (1983a) Characterization of glyoxysomes in yeasts and their transformation into peroxisomes in response to changes in environmental conditions. Arch Microbiol 136:28–38
Zwart KB, Overmars EH, Harder W (1983b) The role of peroxisomes in the metabolism of D-alanine in the yeast Candida utilis. FEMS Microbiol Lett 19:225–231
Zwart KB, Veenhuis M, Harder W (1983c) Significance of microbodies in the metabolism of L-aspartate in C. utilis. FEMS Microbiol Lett 19:273–279
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Veenhuis, M., van der Klei, I.J. & Harder, W. Physiological role of microbodies in the yeast Trichosporon cutaneum during growth on ethylamine as the source of energy, carbon and nitrogen. Arch. Microbiol. 145, 39–50 (1986). https://doi.org/10.1007/BF00413025
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00413025