Abstract
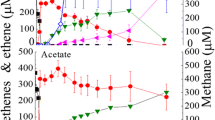
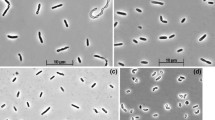
Isovalerate-oxidizing strictly aneerobic bacteria were isolated from marine sediment and sewage sludge in coculture with Desulfovibrio sp. Cells stained Gram positive and behaved Gram positive also in Gram classification with KOH. Isovalerate degradation depended on interspecies hydrogen transfer to syntrophic hydrogen-oxidizing sulfate reducers or methanogens. Isovalerate was the only substrate utilized and was fermented to 3 mol acetate and 1 mol hydrogen per mol substrate. The degradation pathway was studied by enzyme assays in crude cell extracts, and included acetyl-CoA dependent activation of isovalerate, oxidation to methylcrotonyl-CoA and carboxylation to methylgluta-conyl-CoA which is hydrated and cleaved to acetoacetate and acetyl-CoA. Studies with inhibitors and ionophores suggest that energy conservation with this organism depends on either acetate efflux-driven proton symport or on an ion-gradient driven carboxylation mechanism.
Similar content being viewed by others
References
Allison MJ, Bryant MP (1963) Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria. Arch Biochem Biophys 101:262–277
Allison MJ (1969) Biosynthesis of amino acids by ruminal microorganisms. J Anim Sci 29:797–807
Allison MJ (1978) Production of branched-chain volatile fatty acids by certain anaerobic bacteria. Appl Environ Microbiol 35:872–877
American Public Health Association Inc (ed) (1969) Standard methods for the examination of water and wastewater including bottom sediments and sludge. New York, pp 604–609
Barker HA (1981) Amino acid degradation by anaerobic bacteria. Ann Rev Biochem 50:23–40
Bergmeyer HU (1963) Phosphotransacetylase aus Clostridium kluyveri. Biochem Z 338:114–121
Boone O, Bryant MP (1980) Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov. from methanogenic ecosystems. Appl Environ Microbiol 40:626–632
Dimroth P, Hilpert W (1984) Carboxylation of pyruvate and acetyl coenzyme A by reversal of the Na+ pumps oxaloacetate decarboxylase and methylmalonyl-CoA decarboxylase. Biochem 23:5360–5371
Green DE, Mii S, Mahler HR, Bock RM (1954) Studies on the fatty acid oxidizing system of animal tissues. III. Butyryl coenzyme A dehydrogenase. J Biol Chem 206:1–12
Gregersen T (1978) Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur J Appl Microbiol Biotechnol 5:123–127
Harwood CS, Canale-Parola E (1981) Branched-chain amino acid fermentation by a marine Spirochete: strategy for starvation survival. J Bacteriol 148:117–123
Hilpert W, Schink B, Dimroth P (1984) Life by a new decarboxylation-dependent energy conservation mechanism with Na+ as coupling ion. EMBO J 3:1665–1670
Konings WN, Veldkamp H (1983) Energy transduction and solute transport mechanism in relation to environments occupied by microorganisms. In: Slater JH, Whittenbury R, Wimpeny JWT (eds) Microbes in their natural environments. Symp Soc Gen Microbiol 34. Cambridge University Press, Cambridge, pp 153–186
Lynen F, Ochoa S (1953) Enzymes of fatty acid metabolism. Biochim Biophys Acta 12:299–314
Lynen F, Knappe J, Lorch E, Jütting G, Ringelmann E, Lachance JP (1961) Zur biochemischen Funktion des Biotins. II. Reinigung und Wirkungsweise der β-Methyl-Crotonyl-Carboxylase. Biochem Z 335:123–167
Magee CM, Rodeheaver G, Edgerton MT, Edlich RF (1975) A more relible Gram staining technic for diagnosis of surgical infections. Am J Surgery 130:341–346
Massey LK, Sokatch JR, Conrad RS (1976) Branched-chain amino acid catabolism in bacteria. Bacteriol Rev 40:42–54
Mayfield CJ, Inniss WE (1977) A rapid simple method for staining bacterial flagella. Can J Microbiol 23:1311–1313
McInerney MJ, Bryant M, Pfennig N (1979) Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens. Arch Microbiol 122:129–135
McInerney MJ (1986) Anaerobic hydrolysis and fermentation of fats and proteins. In: Zehnder AJB (ed) Environmental microbiology of anaerobes. John Wiley and Sons. New York, in press
Michels PAM, Michels JPJ, Boonstra J, Konings WN (1979) Generation of an electrochemical proton gradient in bacteria by the excretion of metabolic end products. FEMS Microbiol Lett 5:357–364
Pfennig N (1978) Rhodocyclus purpureus gen. nov. and sp. nov., a ring-shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28:283–288
Rose IA, Grunberg-Manago M, Korey SR, Ochoa S (1954) Enzymatic phosphorylation of acetate. J Biol Chem 211:737–756
Schink B, Pfennig N (1982) Propionigenium modestum gen. nov. sp. nov., a new strictly anaerobic, nonsporing bacterium growing on succinate. Arch Microbiol 133:209–216
Schink B (1985) Fermentation of acetylene by an obligate anaerobe, Pelobacter acetylenicus sp. nov. Arch Microbiol 142:295–301
Shelton DR, Tiedje JM (1984) Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic acid. Appl Environ Microbiol 48:840–848
Stieb M, Schink B (1984) A new 3-hydroxybutyrate fermenting anaerobe, Ilyobacter polytropus gen. nov. sp. nov., possessing various fermentation pathways. Arch Microbiol 140:139–146
Stieb M, Schink B (1985) Anaerobic oxidation of fatty acids by Clostridium bryantii sp. nov., a sporeforming, obligately syntrophic bacterium. Arch Microbiol 140:387–390
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Widdel F (1980) Anaerober Abbau von Fettsäuren und Benzoesäuren durch neu isolierte Arten Sulfat-reduzierender Bakterien. Diss Univ Göttingen
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov. sp. nov. Arch Microbiol 129:395–400
Widdel F, Pfennig N (1984) Dissimilatory sulfate- or sulfur-reducing bacteria. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 1. Williams & Wilkins. Baltimore London, pp 663–679
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stieb, M., Schink, B. Anaerobic degradation of isovalerate by a defined methanogenic coculture. Arch. Microbiol. 144, 291–295 (1986). https://doi.org/10.1007/BF00410965
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00410965