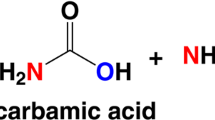
Abstract
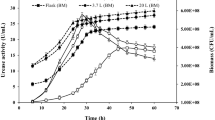
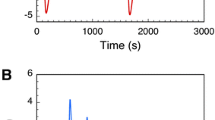
In Arthrobacter oxydans, Klebsiella aerogenes and Sporosarcina ureae, growth with urea as a nitrogen source turned out to be more sensitive to inhibition by EDTA than that with ammonia. The inhibition was overcome by added nickel chloride, but not by other divalent metal ions tested. In A. oxydans the uptake of 63Ni was paralleled by an increase in urease (urea amidohydrolase, EC 3.5.1.5) activity under certain conditions. Following growth with radioactive nickel, urease from this strain was enriched by heat treatment and acetone fractionation. Copurification of 63Ni and urease was observed during subsequent Sephadex gel chromatography. Almost the entire labelling was detected together with the purified enzyme after focusing on polyacrylamide gel. The relative molecular mass of the purified urease was estimated to be 242,000. The pH optimum was 7.6, the K m-value 12.5 mmol/l and the temperature optimum 40°C; heat stability was observed up to 65°C. In presence of 10 mmol/l EDTA the protein-nickel binding remained intact at pH 7; at pH 5 and below, nickel was irreversibly removed with concommitant loss of enzyme activity. The results demonstrated that nickel ions are required for active urease formation in the bacterial strains studied, and that urease from A. oxydans is a nickel-containing enzyme.
Similar content being viewed by others
References
Anderson JA, Kopko F, Siedler AJ, Nohle EG (1969) Purification and properties of urease form Proteus mirabilis. Fed Proc 28:764
Andrews P (1964) Estimation of the molecular weight of proteins by Sephadex gel filtration. Biochem J 91:222–223
Bartha R, Ordal EJ (1965) Nickel-dependent chemolithotrophic growth of two Hydrogenomonas strain. J Bact 89:1015–1019
Blakeley RL, Treston A, Andrews RK, Zerner B (1982) Nickel(II) promoted ethanolysis and hydrolysis of N-(2-pyridylmethyl) urea. A model for urease. J Am Chem Soc 104:612–614
Blakeley RL, Dixon NE, Zerner B (1983) Jack bean urease. VII. Light scattering and nickel(II) spectrum. Biochim Biophys Acta 744:219–229
Cook AR (1976) Urease activity in the rumen of sheep and the isolation of ureolytic bacteria. J Gen Microbiol 92:32–48
Diekert G, Ritter M (1983) Purification of the nickel carbon monoxide dehydrogenase of Clostridium thermoaceticum. FEBS Lett 151:41–44
Diekert G, Graf EG, Thauer RK (1979) Nickel requirement for carbon monoxide dehydrogenase formation in Clostridium pasteurianum. Arch Microbiol 122:117–120
Diekert G, Weber B, Thauer RK (1980) Nickel dependence of factor F430 content in Methanobacterium thermoautotrophicum. Arch Microbiol 127:273–278
Dixon NE, Gazzola C, Blakeley RL, Zerner B (1975) Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc 87:4131–4133
Dixon NE, Blakeley RL, Zerner B (1980a) Jack bean urease (EC 3.5.1.5). 1. A simple dry ashing procedure for the determination of trace metals in proteins. The nickel content of urease. Can J Biochem 58:469–473
Dixon NE, Gazzola C, Asher CJ, Lee DSW, Blakeley RL, Zerner B (1980b) Jack bean urease (EC 3.5.1.5). II. The relationship between nickel enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can J Biochem 58:474–480
Dixon NE, Blakeley RL, Zerner B (1980c) Jack bean urease (EC 3.5.1.5). III. The involvement of active-site nickel ion in inhibition by β-mercaptoethanol, phosphoamidate, and fluoride. Can J Biochem 58:481–488
Dixon NE, Hinds JA, Fihelly A, Gazzola C, Winzor DJ, Blakeley RL, Zerner B (1980d). Jack bean urease (EC 3.5.1.5). IV. The molecular size and the mechanism of inhibition by hydroxamic acids. Spectrophotometric titration of enzymes with reversible inhibitors. Can J Biochem 58:1323–1334
Dixon NE, Riddles PW, Gazzola C, Blakeley RL, Zerner B (1980e). Jack bean urease (EC 3.5.1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, N-methyl-urea, and related compounds. Can J Biochem 58:1335–1344
Drake HL (1982) Occurrence of nickel in carbon monoxide dehydrogenase from Clostridium pasteurianum and Clostridium thermoaceticum. J Bact 149:561–566
Ellefson WL, Whitman WG, Wolfe RS (1982) Nickel containing factor F430: Chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci USA 79:3707–3710
Fishbein WN (1969) A sensitive and non-inhibitory catalytic gel stain for urease. In: Fifth international symposium on chromatography and electrophoresis. Ann Arbor-Humphrey Sci Publ, Ann Arbor London, pp 238–242
Friedrich B, Magasanik B (1977) Urease of Klebsiella aerogenes: Control of its synthesis by glutamine synthetase. J Bact 131:446–452
Friedrich CG, Schneider K, Friedrich B (1982) Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bact 152:42–48
Gordon WR, Schwemmer SS, Hillman WS (1978) Nickel and the metabolism of urea by Lemna paucicostata Hegelm. 6746. Planta 140:265–268
Graf EG, Thauer RK (1981) Hydrogenase from Methanobacterium thermoautotrophicum, a nickel containing enzyme. FEBS Lett 136:165–169
Hasnain SS, Piggott B (1983) An EXAFS study of jack bean urease, a nickel metalloenzyme. Biochim Biophys Res Comm 112:279–283
Kaltwasser H, Frings W (1980) Transport and metabolism of nickel in microorganisms. In: Nriagu JO (ed) Nickel in the environment. John Wiley, New York, pp 463–493
Kaltwasser H, Schlegel HG (1966) NADH-dependent coupled enzyme assay for urease and other ammonia-producing systems. Anal Biochem 16:132–138
Kaltwasser H, Krämer J, Conger WR (1972) Control of urease formation in certain aerobic bacteria. Arch Microbiol 81:178–196
Kamel MY, Hamed RR (1975) Aerobacter aerogenes PRL-R3 urease. Purification and properties. Acta Biol Med Germ 34:971–979
Kirchgessner M, Schnegg A (1980) Biochemical and physiological effects of nickel deficiency. In: Nriagu JO (ed) Nickel in the environment. John Wiley, New York, pp 1–27
Klucas RV, Hanus FJ, Russell SA, Evans HJ (1983) Nickel a micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci USA 80:2253–2257
König C, Kaltwasser H, Schlegel HG (1966) Die Bildung von Urease nach Verbrauch der äußeren N-Quelle bei Hydrogenomonas H16. Arch Microbiol 53:231–241
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
MacKay EM, Pateman JA (1980) Nickel requirement of a urease deficient mutant in Aspergillus nidulans. J Gen Microbiol 116:249–251
MacKay EM, Pateman JA (1982) The regulation of urease activity in Aspergillus nidulans. Biochem Gen 20:763–776
McDonald JA, Speeg KV, Champell JW (1972) Urease: A sensitive and specific radiometric assay. Enzymology 42:1–9
Polacco JC (1977a) Nitrogen metabolism in soybean tissue culture. II. Urea utilisation and urease synthesis require Ni2+. Plant Physiol 59:827–830
Polacco JC (1977b) Is nickel a universal component of plant ureases? Plant Sci Lett 10:249–255
Radola BJ (1980) Ultradünnschicht-isoelektrische Fokussierung. Gesellschaft Deutscher Chemiker, Fortbildungskurs Technische Universität, München
Ragsdale SW, Clark JE, Ljungdahl LG, Lundie LL, Drake HL (1983) Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem 258:2364–2369
Rees TAV, Bekheet IA (1982) The role of nickel in urea assimilation by algae. Planta 156:385–387
Repaske R, Repaske AC (1976) Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol 32:585–591
Romano N, Tolone G, LaLicata R, Ajello F (1979) Urease activity of Ureaplasma urealyticum: some properties of the enzyme. Microbiologica 2:357–367
Schneider J (1984) Nachweis von Nickel in Urease aus Arthrobacter oxydans. Dissertation, Saarbrücken
Soeder CJ, Engelmann G (1984) Nickel requirement in Chlorella emersonii. Arch Microbiol 137:85–87
Spears JW, Hatfield EE (1978) Nickel for ruminants. I. Influence of dietary nickel on ruminal urease activity. J Animal Sci 47:1345–1349
Spears JW, Smith CJ, Hatfield EE (1977) Rumen bacterial urease requirement for nickel. J Dairy Sci 60:1073–1076
Sumner JB (1926) The isolation and cristallisation of the enzyme urease. J Biol Chem 69:435–441
Sumner JB, Gralen N, Eriksson-Quensel JB (1938) The molecular weight of ureases. J Biol Chem 125:37–44
Tabillion R, Kaltwasser H (1977) Energieabhängige 63Nickel-aufnahme bei Alcaligenes eutrophus H1 und H16. Arch Microbiol 113:145–151
Tabillion R, Weber F, Kaltwasser H (1980) Nickel requirement for chemolithotrophic growth in hydrogen-oxidizing bacteria. Arch Microbiol 124:131–136
Thauer RK (1982) Nickel tetrapyrroles in methanogenic bacteria: Structure function, and biosynthesis. Zbl Bakt Hyg I. Abt Orig C 3:265–270
Thauer RK, Brandis-Heep A, Diekert G, Gilles HH, Graf EG, Jaenchen R, Schönheit P (1983) Drei neue Nickelenzyme aus anaeroben Bakterien. Naturwissenschaften 70:60–64
Varner JE (1960) Urease. In: Boyer PD, Lardy H, Myrbäck K (eds) The enzymes, vol 4. Academic Press, New York, pp 247–256
Winkler RG, Polacco JC, Eskew DL, Welch M (1983) Nickel is not required for apourease synthesis in soybean seeds. Plant Physiol 72:263
Zorn C, Dietrich R, Kaltwasser H (1982) Regulation by repression of urease biosynthesis in Proteus rettgeri. Z Allg Microbiol 22:199–205
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Dr. H.-G. Schlegel on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Schneider, J., Kaltwasser, H. Urease from Arthrobacter oxydans, a nickel-containing enzyme. Arch. Microbiol. 139, 355–360 (1984). https://doi.org/10.1007/BF00408379
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00408379